Question: H 2 S from cigarette smoke was collected by bubbling smoke through aqueous NaOH and measured with a sulfide ion-selective electrode. Standard additions of volume
H2S from cigarette smoke was collected by bubbling smoke through aqueous NaOH and measured with a sulfide ion-selective electrode. Standard additions of volume VS containing Na2S at concentration +cS = 1.78 mM were then made to V0 = 25.0 mL of unknown and the electrode response, E, was measured.
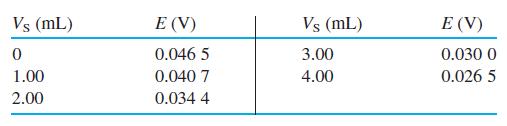
From a separate calibration curve, it was found that β = 0.985 in Equation 14-14. Using T =298.15 K and n = -2 (the charge of S2-), prepare a standard addition graph with Equation 14-15 and find the concentration of sulfide in the unknown.
Vs (mL) E (V) Vs (mL) E (V) 0.046 5 3.00 0.030 0 1.00 0.040 7 4.00 0.026 5 2.00 0.034 4
Step by Step Solution
3.55 Rating (162 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


