Question: This problem can be worked with Equations 18-6 on a calculator or with the spreadsheet in Figure 18-5. Transferrin is the iron-transport protein found in
This problem can be worked with Equations 18-6 on a calculator or with the spreadsheet in Figure 18-5. Transferrin is the iron-transport protein found in blood. It has a molecular mass of 81 000 and carries two Fe3+ ions. Desferrioxamine B is a chelator used to treat patients with iron overload (Box 11-1). It has a molecular mass of about 650 and can bind one Fe3+. Desferrioxamine can take iron from many sites within the body and is excreted (with its iron) through the kidneys. Molar absorptivities of these compounds (saturated with iron) at two wavelengths are given in the table. Both compounds are colorless (no visible absorption) in the absence of iron.

(a) A solution of transferrin exhibits an absorbance of 0.463 at 470 nm in a 1.000-cm cell. Calculate the concentration of transferrin in milligrams per milliliter and the concentration of bound iron in micrograms per milliliter.
(b) After adding desferrioxamine (which dilutes the sample), the absorbance at 470 nm was 0.424, and the absorbance at 428 nm was 0.401. Calculate the fraction of iron in transferrin and the fraction in desferrioxamine. Remember that transferrin binds two iron atoms and desferrioxamine binds only one.
Equation 18-6
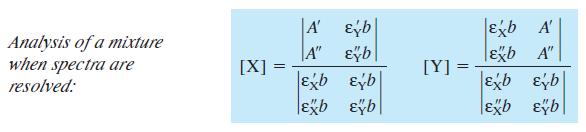
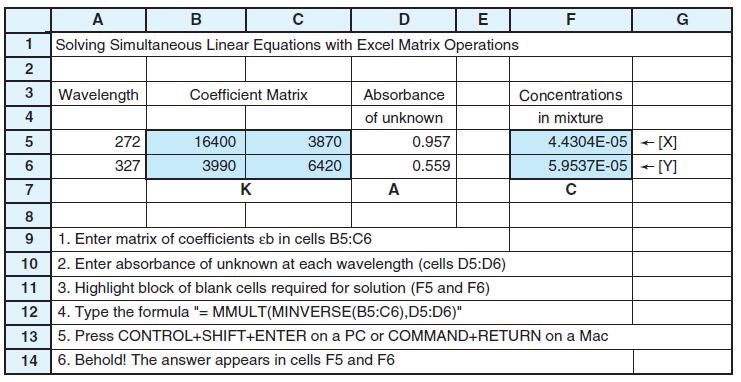
Figure 18-5
E(M cm) A(nm) Transferrin Desferrioxamine 428 3 540 2 730 470 4 170 2 290
Step by Step Solution
3.37 Rating (166 Votes )
There are 3 Steps involved in it
From Lambert Beers Law A C l 1 where is molar absorption coefficient C is molar concentration l is p... View full answer

Get step-by-step solutions from verified subject matter experts


