This problem can be worked by calculator or with the spreadsheet in Figure 18-5. Consider compounds X
Question:
This problem can be worked by calculator or with the spreadsheet in Figure 18-5. Consider compounds X and Y in the example labeled "Analysis of a Mixture, Using Equations 18-6" in Section 18-1. Find [X] and [Y] in a solution whose absorbance is 0.233 at 272 nm and 0.200 at 327 nm in a 0.100-cm cell.
Figure 18-5
![DE 1 Solving Simultaneous Linear Equations with Excel Matrix Operations A в F G Wavelength Coefficient Matrix Absorbance Concentrations 4. of unknown in mixture 5 16400 4.4304E-05 [X] -[Y] 272 3870 0.957 327 3990 6420 0.559 5.9537E-05 7 K A 9 1. Enter matrix of coefficients eb in cells B5:C6](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/8/0/1/2685ee0f5b4c96261591801261927.jpg)
Equation 18-6
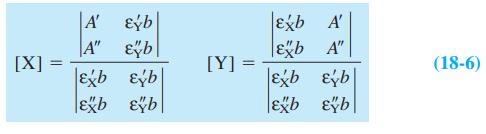
Transcribed Image Text:
DE 1 Solving Simultaneous Linear Equations with Excel Matrix Operations A в F G Wavelength Coefficient Matrix Absorbance Concentrations 4. of unknown in mixture 5 16400 4.4304E-05 [X] -[Y] 272 3870 0.957 327 3990 6420 0.559 5.9537E-05 7 K A 9 1. Enter matrix of coefficients eb in cells B5:C6 10 2. Enter absorbance of unknown at each wavelength (cells D5:D6) 11 3. Highlight block of blank cells required for solution (F5 and F6) 12 4. Type the formula "= MMULT(MINVERSE(B5:C6),D5:D6)" 5. Press CONTROL+SHIFT+ENTER on a PC or COMMAND+RETURN on a Mac 6. Behold! The answer appears in cells F5 and F6 13 14
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (21 reviews)
Putting b 0100 cm into the determinants giv...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
An egg, which for the purposes of this problem can be assumed to be a 5-cm-diameter sphere having the thermal properties of water, is initially at a temperature of 4?C. It is immersed in boiling...
-
Refer to Multiple-Concept Example 5 to review a method by which this problem can be solved. You are driving your car, and the traffic light ahead turns red. You apply the brakes for 3.00 s, and the...
-
Useful background for this problem can be found in Multiple-Concept Example 2. On a spacecraft two engines fire for a time of 565 s. One gives the craft an acceleration in the x direction of ax =...
-
Is a flat-rate or flat-fee system more efficient for pricing scarce water? Why?
-
Lynn Company acquires the land and building owned by Noble Company. What types of costs may be incurred to make the asset ready for its intended use if Lynn Company wants to use (a) Only the land,...
-
Assume for Questions 5 through 9 that the state of Exuberance issued $10,000,000 of 5%, 20-year refunding bonds in 20X5 at par. If the state placed $12,000,000 (the $10,000,000 from the advance...
-
Analizar los papeles importantes del marketing interno y de la administracin de la experiencia con los consumidores en las organizaciones de servicios .
-
Jana Crebs is a contractor for the construction of large office buildings. At the beginning of 2011, three buildings were in progress. The following data describe the status of these buildings at the...
-
During December, Far West Services makes a $3,200 credit sale. The state sales tax rate is 6% and the local sales tax rate is 25% (Note: the sales tax amount is in addition to the credit sale...
-
Kalyan Singhal Corp. makes three products, and it has three machines available as resources as given in the following LP problem: Maximize contribution = 4X1 + 4X2 + 7X3 Subject to: 1X1 + 7X2 + 4X3 ...
-
Color and absorption spectra. Color Plate 15 shows colored solutions and their spectra. From Table 17-1, predict the color of each solution from the wavelength of maximum absorption. Do observed...
-
Figure 18-7 is a Scatchard plot for the addition of 0-20 nM antigen X to a fixed concentration of antibody P (P 0 = 10 nM). Prepare a Scatchard plot from the data in the table and find K for the...
-
TELUS Corporation is one of Canadas largest telecommunications companies and provides both products and services. Its shares are traded on the Toronto and New York stock exchanges, and its credit...
-
10.1 Learning Outcomes: Describe managers' appropriate use of power and influence. Identify traits and characteristics of successful leaders. Identify behaviors of successful leaders 10.2 Action...
-
Suppose Ron went to Rio de Janeiro in 2019 when the dollar was worth 4 reals. The price of a cup of coffee at Starbucks in the US was $4, so when Ron converted $4 into reals he had 16 reals, which...
-
The expected annual net income is $200,000; the average investment is $800,000; and depreciation expense is $50,000. Calculate the annual rate of return.
-
How do you define humanities?
-
Write the types of partners ?
-
Why should contracts be in writing, even if the law doesn't require it?
-
Grace is training to be an airplane pilot and must complete five days of flying training in October with at least one day of rest between trainings. How many ways can Grace schedule her flying...
-
Calculate the pH at each of the following points in the titration of 50.00 mL of 0.010 0 M NaOH with 0.100 M HCl. Volume of acid added: 0.00, 1.00, 2.00, 3.00, 4.00, 4.50, 4.90, 4.99, 5.00, 5.01,...
-
Calculate the pH at each point listed for the titration of 50.0 mL of 0.050 0 M formic acid with 0.050 0 M KOH. The points to calculate are V b = 0.0, 10.0, 20.0, 25.0, 30.0, 40.0, 45.0, 48.0, 49.0,...
-
Calculate the pH at each point listed for the titration of 100.0 mL of 0.100 M cocaine (Section 8 4, K b = 2.6 10 -6 ) with 0.200 M HNO 3 . The points to calculate are V a = 0.0, 10.0, 20.0, 25.0,...
-
What is the present value of $500 invested each year for 10 years at a rate of 5%?
-
GL1203 - Based on Problem 12-6A Golden Company LO P2, P3 Golden Corp.'s current year income statement, comparative balance sheets, and additional information follow. For the year, (1) all sales are...
-
A project with an initial cost of $27,950 is expected to generate cash flows of $6,800, $8,900, $9,200, $8,100, and $7,600 over each of the next five years, respectively. What is the project's...

Study smarter with the SolutionInn App


