Color and absorption spectra. Color Plate 15 shows colored solutions and their spectra. From Table 17-1, predict
Question:
Color and absorption spectra. Color Plate 15 shows colored solutions and their spectra. From Table 17-1, predict the color of each solution from the wavelength of maximum absorption. Do observed colors agree with predicted colors?
Table 17-1
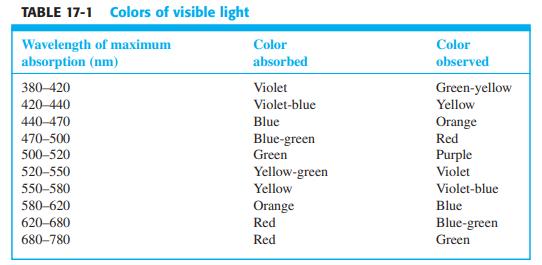
TABLE 17-1 Colors of visible light Wavelength of maximum absorption (nm) Color Color absorbed observed 380-420 Violet Green-yellow 420-440 Violet-blue Yellow 440-470 Blue Orange 470-500 500-520 Red Blue-green Green Purple 520-550 Yellow-green Violet 550-580 Yellow Violet-blue 580-620 Orange Blue 620-680 Red Blue-green 680-780 Red Green
Step by Step Answer:
Curve A B D E F Absorption peak nm 760 ...View the full answer



Related Video
Using a few basic physics principles, you can impress your friends with a trick that makes a bottle disappear. For this experiment, you will need a mini plastic bottle, glycerin, a glass, and a funnel. First, open the bottle and pour some glycerin into it, then close the bottle tightly. Next, pour some water into the glass using a funnel. Place the mini bottle into the glass of water and it will look normal. This is because light travels through air faster than it travels through the glass and water, allowing our eyes to see the bottle inside the glass. However, when you fill the mini bottle with more glycerin, and pour glycerin into the glass, then put the glycerin filled bottle into the glass with glycerin in it. Half of the bottle that is submerged in the glycerin will become invisible as the light travels through glass and glycerin at the same speed, thus it does not bend and no refraction takes place, making the bottle invisible. This happens because both glass and glycerin have almost the same refractive index, which causes the speed of light to be the same in both mediums, causing no bending of light and making the bottle disappear.
Students also viewed these Chemical Engineering questions
-
The photograph of upconversion in Color Plate 19 shows total internal reflection of the blue ray inside the cuvet. The angle of incidence of the blue ray on the wall of the cuvet is 55. We estimate...
-
The solutions shown here each have an absorption spectrum with a single absorption peak like that shown in Figure 23.26. What color does each solution absorb most strongly? [Section 23.5]
-
Predict the positions of the absorption bands in the IR spectra for the carbonyl groups of thesecompounds. HO. b) CH,CH-CHCH (p
-
One major concern about the future is that water scarcity will grow, particularly in arid regions where precipitation levels may be reduced by climate change. Will our institutions provide for an...
-
In a recent newspaper release, the president of Downs Company asserted that something has to be done about depreciation. The president said, Depreciation does not come close to accumulating the cash...
-
Which of the following should be reported as expenditures in a county General Fund? (1) Reimbursement of a Special Revenue Fund for General Fund expenditures inadvertently paid for from and recorded...
-
Explicar la funcin de las siete P en la mezcla de marketing de los servicios .
-
Kilgore's Deli is a small delicatessen located near a major university. Kilgore's does a large walk-in carry-out lunch business. The deli offers two luncheon chili specials, Wimpy and Dial 911. At...
-
The current assets and current liabilities sections of the balance sheet of Pharoah Co. appear as follows. Cash Accounts receivable Less: Allowance for doubtful accounts Inventory Prepaid expenses...
-
Figure shows the circuit diagram of a square-law modulator. The signal applied to the non linear devices is relatively weak, such that it can be represented by a square law: v2 (t) = a1v1 (t) + a2v21...
-
Characteristic orange light produced by sodium in a flame is due to an intense emission called the sodium D line, which is actually a doublet, with wavelengths (measured in vacuum) of 589.157 88 and...
-
This problem can be worked by calculator or with the spreadsheet in Figure 18-5. Consider compounds X and Y in the example labeled "Analysis of a Mixture, Using Equations 18-6" in Section 18-1. Find...
-
Entertainment Centre Ltd. reported the following data at March 31, 2014, with amounts adapted and in thousands: You are the CFO responsible for reporting Entertainment Centre Ltd. (ECL) results. Use...
-
1. Identify an industry that competes internationally (i.e., fast food, clothing, sportswear, automotive, etc). All your companies must be from ONE Industry (you cannot discuss Taco Bell and Nike)....
-
A research article on " Leadership in Project Management: Cultivating Strong Employee-Employer Bonds" shows major findings on why big companies fail in leadership skill practice. How they can...
-
Discuss and Identify the current types of stock, such as common or preferred stock, currently issued, and outstanding. Include a narrative description along with the values and number of shares found...
-
The organization we intend to study is Local Point, a student cafeteria run by UW Housing & Food Services. Our team would like to figure out how to utilize modern technology and rational...
-
Briefly summarize the Coase Theorem (include the 3 key conditions). List the major types of approaches government typically takes to deal with negative externalities. Suppose the demand for...
-
Why is the agreement considered especially important?
-
Figure displays a 12.0 V battery 3 four uncharged capacitors of capacitances C1 = 4.00F, C2 = 6.00F, and C3 = 3.00F. The switch is thrown to the left side until capacitor 1 is fully charged. Then the...
-
Consider the following reaction: 2 NO(g) + 2 H2(g) -- N2(g) + 2 H2O(g) (a) The rate law for this reaction is first order in H2 and second order in NO. Write the rate law. (b) If the rate constant for...
-
Consider the following reaction: CH3Br(aq) + OH (aq) -- CH3OH(aq) + Br(aq) The rate law for this reaction is first order in CH3Br and first order in OH. When [CH3Br] is 5.0 10-3 M and [OH] 0.050 M,...
-
The reaction between ethyl bromide (C2H5Br) and hydroxide ion in ethyl alcohol at 330 K, C2H5Br(alc) + OH (alc) -- C2H5OH(I) + Br(aIc), is first order each in ethyl bromide and hydroxide ion. When...
-
*please calculate irr in excel
-
Which of the following would not be a period cost? Research and development Direct materials Office supplies Advertising costs
-
\ table [ [ Activity Cost Pool,Activity Measure,Total Cost,Total Activity ] , [ Machining , Machine - hours,$ 3 3 0 , 0 0 0 , 1 5 , 0 0 0 MHs ] , [ Machine setups,Number of setups,$ 3 0 0 , 0 0 0 , 5...

Study smarter with the SolutionInn App


