Predict the positions of the absorption bands in the IR spectra for the carbonyl groups of thesecompounds.
Question:
Predict the positions of the absorption bands in the IR spectra for the carbonyl groups of thesecompounds.
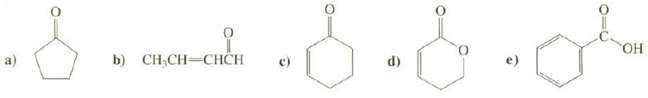
Transcribed Image Text:
HO. b) CH,CH-CHCH (p
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
a The carbonyl group of this ketone is part of a five membered ring so the band will be s...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
62+ Reviews
349+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the positions of the major absorption bands in the IR spectra of thesecompounds: CH30 CH-NH, a) CH3CH=CHCCH; c) b) C-H NO, e) CH-CH,C3- d) CH,CH,CH,OH CH3
-
Indicate the positions of the absorption bands and any other noteworthy features in the hydrogen region of the IR spectra of thesecompounds: CH3 ) C,CH b) NH2 d) CH2=CHCH,CH,OH f) CH,CH,CH,CH e)...
-
Predict the key IR absorption bands whose presence would allow each compound in pairs (a), (c), (d), (e), (g), and (i) from Problem 2.46 to be distinguished from each other. In problems 2.46 (a) (c)...
-
On the cost of goods manufactured schedule, depreciation onfactory equipment... A. is not listed because it is not a product cost. B. is not an inventoriable cost. C. is not listed because it is...
-
From experience and/or research, explain what you think are the best practices when training, developing and providing base salaries for expatriates. Describe the differences that people may see in...
-
Suppose Becky has her choice of $10,000 at the end of each month for life or a single prize of $1.5 million. She is 35 years old and her life expectancy is 40 more years. (i) Find the present value...
-
A paired difference experiment with n = 30 pairs yielded T+ = 354. a. Specify the null and alternative hypotheses that should be used in conducting a hypothesis test to determine whether the...
-
Alvarez Company produces various component parts used in the automotive industry. The sales budget for the first eight months of 2010 shows the following projections: Inventory on December 31 of the...
-
"Bellingham Company produced 15,000 units of product that required 4 standard direct labor hours per unit. The standard variable overhead cost per unit is $0.90 per direct labor hour. The actual...
-
What is the required bandwidth for the following cases if we need to send 4000 bps? Let d = 1. a. ASK b. FSK with 2f = 4 KHz c. QPSK d. 16-QAM
-
The exhaust from a poorly maintained automobile may contain a wide variety of different hydrocarbon pollutants. Why is the 3000 to 2900 cm1 region a good place to monitor the amount of these...
-
Explain how IR spectroscopy could be used to distinguish between thesecompounds: CH,COCH, and COCH,CH, a) CH,CH,CH,CCH, and CH,CH,CH,CH,CH b) c) CH;CH-CHOCH, and CH,CH-CHCH,COH
-
(a) To calculate the "capacitance" of an isolated sphere, evaluate the result we obtained in Example 26.4 in the limit that \(R_{2}\) goes to infinity. (b) What is the capacitance of the spherical...
-
The force vector F has a magnitude of F = 385 lb and acts at point A at an angle 0 = 17 with respect to vertical as shown. The force F is balanced by the tension forces parallel to the two rods AC...
-
D1 Justify the use of a specific moulding technique for the manufacture of a given product
-
the igniter is made of a wire with paper tape holding it . In the head of the igniter is a very thin wire surrounded by pyrotechnic material. Pressing the second switch allows more current to flow...
-
Problem - Process Costing Atticus Electronics produces travel batter pack chargers. The company uses a process costing system. The following information pertains to operations for November Percentage...
-
B . what is the wavespeed? C . What is the frequency? D . What is the wave number? E . At t = 0 . 4 9 s , what is the diplacement of the string at x = 5 . 2 m ?
-
discuss a variety of issues facing operations managers in sports businesses;
-
A test car is driven a fixed distance of n miles along a straight highway. (Here n Z+.) The car travels at one mile per hour for the first mile, two miles per hour for the second mile, four miles...
-
What likely happened to the four-firm concentration ratio in the airline industry when United and Continental Airlines merged? What likely happened to the eight-firm concentration ratio?
-
1, 3-Cyclopentadiene undergoes thermal polymerization to yield a polymer that has no double bonds in the chain. On strong heating, the polymer breaks down to regenerate cyclopentadiene. Propose a...
-
When styrene, C6H5CH = CH2, is copolymerized in the presence of a few percent p-divinyl benzene, a hard, insoluble, cross-linked polymer is obtained. Show how this cross-linking of polystyrene chains...
-
Poly (ethylene glycol), or Carbowax, is made by anionic polymerization of ethylene oxide using NaOH as catalyst. Propose amechanism. +0-CH2CH2t Polylethylene glycol)
-
Selected comparative financial statement data for DAS inc. Balance Sheet (En milliers de dollars) 2017 2018 Assets Assets CT - Cash 41.63 47.5 - Accounts Receivable 64.2 72.6 - inventories 969.7...
-
please help!! One chance at turning in!!! 16 rows! I'd highly appreicate it I am unsure what information you need... I provided all Current Attempt in Progress Mike Greenberg opened Grouper Window...
-
Blue Ridge Marketing Inc. manufactures two products, A and B . Presently, the company uses a single plantwide factory overhead rate for allocating overhead to products. However, management is...

Study smarter with the SolutionInn App


