Predict the positions of the major absorption bands in the IR spectra of thesecompounds: CH30 CH-NH, a)
Question:
Predict the positions of the major absorption bands in the IR spectra of thesecompounds:
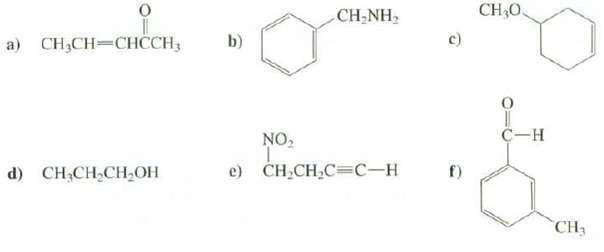
Transcribed Image Text:
CH30 CH-NH, a) CH3CH=CHCCH; c) b) C-H NO, e) CH-CH,C3С-н d) CH,CH,CH,OH CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
a CH 31003000 CH 30002850 CO 16951675 CC 16601640 b NH2 34003250 two bands CH ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the positions of the absorption bands in the IR spectra for the carbonyl groups of thesecompounds. HO. b) CH,CH-CHCH (p
-
Predict the major characteristic IR absorption bands that would be given by each of the following compounds: a. b. c. d. e. f. CH2 CHCH2CH COCH2CH3 CH3CH2CCH2CH2NH2 csco CH2COH
-
Predict the key IR absorption bands whose presence would allow each compound in pairs (a), (c), (d), (e), (g), and (i) from Problem 2.46 to be distinguished from each other. In problems 2.46 (a) (c)...
-
You have recently been appointed as assistant to the financial controller of Chaffinch plc, a manufacturing company. He has asked you to help with the finalisation of the financial statements for the...
-
The Connecting Company uses the percent of sales method of accounting for uncollectible accounts receivable. During the current year, the following transactions occurred: Sept 7 Connecting Company...
-
In order to upgrade its equipment, Simon Reilly Chemicals (SRC) borrowed $2.8 million at 6.4% compounded quarterly for 20 years. After making 18 quarterly payments of $62,297.39, SRC plans to...
-
Suppose you want to test a hypothesis that two treatments,A and B, are equivalent against the alternative hypothesis that the responses for A tend to be larger than those for B. You plan to use a...
-
Discuss why organizational design and communication flow are so closely related.
-
A six-year 6.1% semi-annual coupon bond has a yield to maturity of 10% and a Macaulay duration of 10.014 (in half-years). a. What is the modified duration in years? b. If the yield increases by 25...
-
Write and test a program to simulate the flow diagram of CSMA/CD in Figure 12.13. Figure 12.13 Flow diagram for the CSMA/CD Station has a frame to send K= 0 Legend T Frame average transmission time...
-
Explain how IR spectroscopy could be used to distinguish between thesecompounds: CH,COCH, and COCH,CH, a) CH,CH,CH,CCH, and CH,CH,CH,CH,CH b) c) CH;CH-CHOCH, and CH,CH-CHCH,COH
-
Explain how IRD spectroscopy could be used to distinguish between these compounds: CH;CH,C=CH a) CH,CH,CH=CH; and CH3 H- b) CH, nd CH3 CCH3 CH c) and d) CH.CH,CH,C,NH, and CH.CH,NHCH,CH,
-
A company reports the following: a. Determine the company's earnings per share on common stock. b. Determine the company's price-earnings ratio.objs. 2, 3 Net income Preferred dividends Shares of...
-
Consider the following account balances (in thousands) for the Shaker Corporation In the Dec 31.2021 Cash $200,000 and Capital $2,000,000 and Retained earnings $1,500,000 The balances of raw...
-
Unless otherwise stated, assume gravitational acceleration g = 9.81 m/s and the density of water to be 1000 kg/m. Unless otherwise stated, give all numerical answers to 3 significant figures, such as...
-
The purpose of this installment is to classify stock, bond, and mutual fund investments, explore tools for their evaluation and select these securities based on your investment philosophy and goals....
-
Jackson County Senior Services is a nonprofit organization devoted to providing essential services to seniors who live in their own homes within the Jackson County area. Three services are provided...
-
Caldwell (2003) explores differences between the roles of leaders and managers. "Leaders...envision, initiate, or sponsor strategic change of a far-reaching or transformational nature. In contrast,...
-
identify a range of performance measures that can be used by managers in sports organisations and discuss the implementation of measures (and associated problems);
-
Find the radius of convergence of? 1.2.3 1.3.5 (2n-1) r2n+1 -1
-
Automated Clearing Houses (ACHs) process both private payments, such as automatic deposits to wage-earners bank accounts, and public payments, such as direct deposits of Social Security stipends. The...
-
Glyptal is a highly cross-linked thermosetting resin produced by heating glycerol and phthalic anhydride (l, 2-benzcnedicarboxylic acid anhydride). Show the structure of a representative segment of...
-
Melmac, a thermosetting resin often used to make plastic dishes, is prepared by heating melamine with formaldehyde. Look at the structure of Bakelite shown in Section 31.5, and then propose a...
-
Epoxy adhesives arc cross-linked resins prepared in two steps. The first step involves S N 2 reaction of the disodium salt of bisphenol A with epichiorohydrin to form a low-molecular-weight...
-
Suppose the S&P 500 currently has a level of 960. One contract of S&P 500 index futures has a size of $250 S&P 500 index. You wish to hedge an $800,000-portfolio that has a beta of 1.2. (A)In order...
-
Exhibit 4.1 The balance sheet and income statement shown below are for Koski Inc. Note that the firm has no amortization charges, it does not lease any assets, none of its debt must be retired during...
-
Haley is 57 years of age. She is planning for future long-term care needs. She knows that yearly nursing home costs in her area are currently $69,000, with prices increased by 5 percent annually....

Study smarter with the SolutionInn App


