Explain how IRD spectroscopy could be used to distinguish between these compounds: CH;CH,C=CH a) CH,CH,CH=CH; and CH3
Question:
Explain how IRD spectroscopy could be used to distinguish between these compounds:
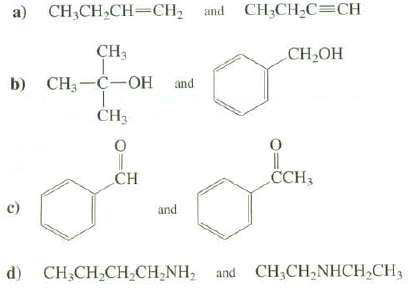
Transcribed Image Text:
CH;CH,C=CH a) CH,CH,CH=CH; and CH3 СH-ОН b) CH, —С—ОН nd CH3 CCH3 CH c) and d) CH.CH,CH,Cн,NH, and CH.CH,NHCH,CH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (19 reviews)
a 1Butyne has absorptions at 3300 cm1 CH and 215021 00 cm1 CC that are not present in the spect...View the full answer

Answered By

Patrick Busaka
I am a result oriented and motivated person with passion for challenges because they provide me an opportunity to grow professionally.
5.00+
38+ Reviews
58+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Explain how IR spectroscopy could be used to distinguish between thesecompounds: CH,COCH, and COCH,CH, a) CH,CH,CH,CCH, and CH,CH,CH,CH,CH b) c) CH;CH-CHOCH, and CH,CH-CHCH,COH
-
Explain how IR spectroscopy could be used to distinguish between thesecompounds: and b) and NH2 d d) and and e) ) and
-
Explain how UV spectroscopy could be used to distinguish between thesecompounds. and a) b) and c) and HO and d)
-
Please do this two questions, please step by step 9. 11. 0/1 points | Previous Answers SEssCalcET1 12.1.020. Calculate the iterated integral. SS 5xyx + y dy dr = || Need Help? Read It Submit Answer...
-
Matt and Grace own a small supermarket in a rural town with a large and growing elderly population. Because of their remote location, they don't have any competition from the large chain stores. A...
-
Clark and Lana take a 30-year home mortgage of $121,000 at 7.8%, compounded monthly. They make their regular monthly payments for 5 years, then decide to pay $1000 per month. (a) Find their regular...
-
Specify the test statistic and the rejection region for the Wilcoxon signed rank test for the paired difference design in each of the following situations: a. n = 30, a = .10 H,:Two probability...
-
The price of Garden Designs, Inc. is now $85. The company pays no dividends. Sean Perth expects the price four years from now to be $125 a share. Should Sean buy Garden Designs if he wants a 15...
-
Please try to slove all parts
-
The Boston Fire Department receives 911 calls at a mean rate of 1.6 calls per hour (Mass.gov website, November 2012). Suppose the number of calls per hour follows a Poisson probability distribution....
-
Predict the positions of the major absorption bands in the IR spectra of thesecompounds: CH30 CH-NH, a) CH3CH=CHCCH; c) b) C-H NO, e) CH-CH,C3- d) CH,CH,CH,OH CH3
-
List the positions of the important absorption bands in the IR spectra of these compounds: b) H,H-CH-C3DN ) -H,H,NH> CCH,CH3 c) CH;CH,COCH;CH,CH3 d)
-
Solve the equation. (x + 1) 2/5 - 3(x + 1) 1/5 + 2 = 0
-
1. What gives stainless steels their good corrosion resistant properties? 2. Which stainless steel is the lowest cost and why? 3. What are some characteristics of Nickel Alloys? 4. What are the 2...
-
Problem 4. Determine the motion of a two-dimensional linear oscillator of potential energy V = kr
-
5 Informatics solutions in the "complex and catastrophic" end of the population-risk spectrum must support which type of services/functions? 1 point Intensive case management Wellness program
-
What are the characteristics of products that Otis Trains produces? What are order qualifiers and winners? Explain at least three advantages and three drawbacks of offshoring to JLPTC. What risks are...
-
Find the angle and length of the resulting vector for the given d and e vectors by the analytical method. After that, find the parameters of the resulting vector for the three vectors. In the answer,...
-
recognise a variety of issues for managers who are responsible for managing performance in sports organisations;
-
On average there are four traffic accidents in a city during one hour of rush-hour traffic. Use the Poisson distribution to calculate the probability that in one such hour there arc (a) No accidents...
-
In the last few years, streaming services have become as much, if not more, popular than cable TV. This popularity has induced companies such as Amazon and Netflix to enter into new markets, like...
-
The polyurethane foam used for home insulation uses methane diphenyl-diisocyanate (MDI) as monomer. The MDI is prepared by acid-catalyzed reaction of aniline with formaldehyde, followed by treatment...
-
Write the structure of a representative segment of polyurethane prepared by reaction of ethylene glycol with MDI.
-
The smoking salons of the Hindenburg and other hydrogen-filled dirigibles of the 1930s were insulated with urea formaldehyde polymer foams. The structure of this polymer is highly cross-linked, like...
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


