The smoking salons of the Hindenburg and other hydrogen-filled dirigibles of the 1930s were insulated with urea
Question:
The smoking salons of the Hindenburg and other hydrogen-filled dirigibles of the 1930s were insulated with urea formaldehyde polymer foams. The structure of this polymer is highly cross-linked, like that of Bakelite (Section 31 .5). Propose astructure.
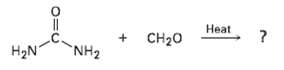
Transcribed Image Text:
Нeat Нeat + CH20 NH2 H2N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
HN i NH ...View the full answer

Answered By

Moses mwangi
With prior writing experience, be sure that I will give a great grade, If not an A+, it will be something close to this. My reviews speaks it all, Try me!!
4.80+
78+ Reviews
157+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a structure that is consistent with each set of 1H NMR data. IR data is provided for some compounds. (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) (m) C4H1OO 6 (ppm) Splitting 1.28 1.35...
-
The structure of poly(vinyl alcohol) is shown below. This polymer cannot be made by polymerizing its monomer. Why not? How could poly(vinyl alcohol) be prepared from poly(vinyl acetate)? --(CH2CH,-...
-
Propose a structure for ether with molecular formula C 7 H 8 O that exhibits the following 13 C NMR spectrum. Carbon NMR 114.0 129.5, 120,714.0 55.1- 159.71 160 120 140 100 100 Chemical shift (ppm)...
-
Fox Erasing has a system of internal control with the following procedures. Match the procedure to the corresponding internal control principle. Procedure Internal Control Principle A. Establish...
-
What are the differences between the general environment and the industry environment? Why are these differences important?
-
This and the next two exercises are based on the test statistic usually used to test a set of J linear restrictions in the generalized regression model where is the GLS estimator. Show that if is...
-
how a researcher used path analysis to study the social origins of distress
-
Given below is a list of account balances for Currie Hospital as of December 31, 2004. Prepare a balance sheet as of December 31, 2004, in good form. AccountBalance Gross plant & equipment...
-
Fleetwood Corp. purchased a mine in 2016 for $500,000 and estimated that 30,000 tons of iron ore could be extracted from it. There was no residual value. The business extracted and sold 2,500 tons of...
-
You have been hired by Gnomeo, Inc., a company that buys and resells miniature garden gnomes. The company started business on January 1, 2019. The chief accountant has asked you to compile a set of...
-
Write the structure of a representative segment of polyurethane prepared by reaction of ethylene glycol with MDI.
-
The polymeric resin used for Merrifield solid-phase peptide synthesis (Section 26.8) is prepared by treating polystyrene with N-(hydroxymethyl) phthalimide and trifluoromethanesulfonic acid, followed...
-
Sharmila Khan is manager of TaxExperts.co.uk, a firm that provides assistance in the preparation of individual tax returns via the Internet. Because of the highly seasonal nature of her business,...
-
Adidas-Consumer Goods STEP ONE: MISSION: Mission statement core message that guides and influences your marketing strategy. Why is this company in business and what is the purpose of their...
-
You have been operating and growing your golf club for the last six (6) years. You are happy with the fact that all revenue streams (and as a result your share value) have continued to increase as...
-
Given the following HTML, write a simple bit of JavaScript code that will DELETE ALL OF THE TAGS ON THE PAGE. Quiz I'm a Heading I'm a paragraph I'm special I'm also a paragraph Footer! HINT: You'll...
-
Your company has been quite successful in sending employees on international assignments. As the HR Manager responsible for selecting such employees, present a report to the management of your...
-
You will be looking at a particular market in the economy. I will assign the market to you arbitrarily. Please look for at the end of this document to identify which market you will be responsible...
-
Discuss why it is important to know the characteristics of your labor needs.
-
Software Solution is family-owned business that has been in operation for more than 15 year. The board of directors is comprised of mainly family members, plus a few professionals such as an...
-
A gas mixture containing equimolar quantities of carbon dioxide and hydrogen is to be reformed by passing it over a catalyst. The pressure in the reformer will be determined by the possibility of...
-
One stereoisomer of 1,1,3,5-tetramethylcyclohexane is 15 kJ/mol (3.7 kcal/mol) less stable than the other. Indicate which isomer is the less stable, and identify the reason for its decreased...
-
One of the following two stereoisomers is 20 kJ/mol (4.9 kcal/mol) less stable than the other. Indicate which isomer is the less stable, and identify the reason for its decreased stability. B
-
Cubane (C8H8) is the common name of a polycyclic hydrocarbon that was first synthesized in the early 1960s. As its name implies, its structure is that of a cube. How many rings are present in cubane?...
-
Estimate the intrinsic value of the stock company ABC. Dividends were just paid at $8 per share and are expected to grow by 5%. You require 20% on this stock given its volatile characteristics. If...
-
Crane, Inc., a resort management company, is refurbishing one of its hotels at a cost of $6,794,207. Management expects that this will lead to additional cash flows of $1,560,000 for the next six...
-
Match each of the following transactions with the applicable internal control principle that is being violated

Study smarter with the SolutionInn App


