Question: Propose a structure for ether with molecular formula C 7 H 8 O that exhibits the following 13 C NMR spectrum. Carbon NMR 114.0 129.5,
Propose a structure for ether with molecular formula C7H8O that exhibits the following13C NMR spectrum.
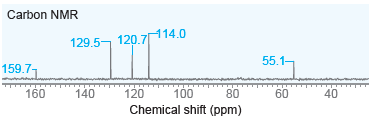
Carbon NMR 114.0 129.5, 120,714.0 55.1- 159.71 160 120 140 100 100 Chemical shift (ppm) 80 80 60 60 40
Step by Step Solution
3.36 Rating (171 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


