How many absorption are expected in the 1 H-NMR spectra of these compounds? CCH; .
Question:
How many absorption are expected in the 1 H-NMR spectra of these compounds?
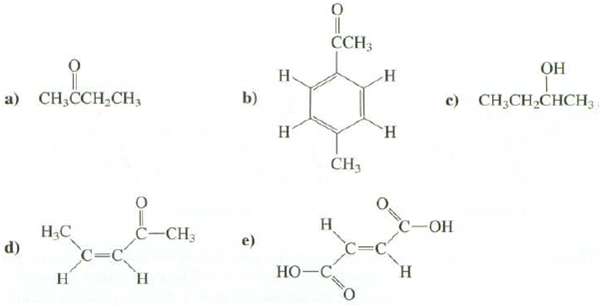
Transcribed Image Text:
CCH; ОН Н. н а) Cн,ССH,CH, c) CH;CH,CHCH3 b) н "н CH3 Н C=C н С -ОН Н.С d) С-СН, e) C= Н Но-С н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (16 reviews)
a The number of absorption signals in the NMR spectrum is equal to the number of different types of ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the 1H.NMR spectra of these compounds include the approximate chemical shift, multiplicity, and integral for each type ofhydrogen. CI b) CH;CHCH; ) C,CH,H c) CH,CH,OCH,CH3 CH2CH2NO2 f)...
-
The following 1H NMR spectra are for four compounds with molecular formula C6H12O2, Identify the compounds. a. b. c. QUESTION CONTINUE TO NEXT PAGE d. 10 (ppm) frequency 10 (ppm) 10 (ppm)
-
NMR spectra for two compounds are given here, together with the molecular formulas. Each compound is a ketone or an aldehyde. In each case, show what characteristics of the spectrum imply the...
-
In determining an employee's net pay, which of the following taxes would be deducted? a. FUTA taxes b. SUTA taxes c. FICA taxes d. All of these choices are correct.
-
At mixing department, all materials are added at the beginning of the process. Lahore and overhead (conversion resources) are added evenly throughout the process. The following information pertains...
-
1. Expand (a + b)6. 2. Expand (x + y)5. 3. Expand (x + h)4.
-
Use Tables VIII, IX, X, and XI of Appendix B to find each of the following F values: a. F,, where vl = 9 and v2 = 6 b. Fnl where v, = 18 and v2 = 14 c. F,,,, where v, = 11 and v2 = 4 d. Fln where v,...
-
Briones Books is concerned about the profitability of its regular dictionaries. Company managers are considering producing only the top-quality, hand-sewn dictionaries with gold-edged pages. Briones...
-
Beach Vacations pays salaries and wages on the last day of each month. Payments made on December 3 1 , 2 0 1 , for amounts incurred during December are shown below. Cumulative amounts paid prior to...
-
Imperial Petroleum plc is a multinational oil and gas company based in the United Kingdom with substantial offshore production and exploration activities. Imperial prepares an annual sustainability...
-
The absorption for the hydrogens of benzene appears 444Hz downfield from TMS on an instrument that operates of 60MHz. (a) Calculate the position of this absorption in units. (b) Calculate the...
-
Predict the approximate chemical shifts for the different hydrogen's in thesecompounds: CI CI a) CH,CH,CH3 b) CH;CHCH3 c) CH,COCH,CH3 d) CH;CHCH2
-
Explain the purpose of the CHECK clause within a CREATE TABLE SQL command. Explain the purpose of the WITH CHECK OPTION in a CREATE VIEW SQL command.
-
Based on the case, Insights Analytics: Technology for a Knowledge Management Program attached . Please explain all 8 points. Explanation of each point should be 300words . Please attach the reference...
-
When women were finally allowed to become pilots of fighter jets, engineers needed to redesign the ejection seats because they had been originally designed for men only. The ejection seats were...
-
What will be the output of the following code snippet? with open ("hello.txt", "w") as f: f.write("Hello World how are you today") with open('hello.txt', 'r') as f: data = f.readlines () for line in...
-
Assume that females have pulse rates that are normally distributed with a mean of p = 72.0 beats per minute and a standard deviation of o = 12.5 beats per minute. Complete parts (a) through (c)...
-
The proposed rates were not in the range the CEO expected given the pricing analysis. The CEO has asked the pricing actuary to verify the total projected loss cost excluding potential large storm...
-
What is the difference between customer satisfaction and service quality?
-
Sue Deliveau opened a software consulting firm that immediately paid $2,000 for a computer. Was this event a transaction for the business?
-
Since the mid-1990s, individuals have been using online auction sites such as eBay, uBid, and Yahoo! to sell off old clothes, collectibles, and other used items that they find when cleaning out...
-
Propose a mechanism for the conversion of fumaroyl acetoacetate to fumarate plus acetoacetate (Problem 29.49).
-
Propose a mechanism for the conversion of acetoacetate to acetyl CoA (Problem 29.49).
-
Design your own degradative pathway. You know the rules (organic mechanisms), and you?ve seen the kinds of reactions that occur in the biological degradation of fats and carbohydrates into acetyl...
-
What is the Breakeven Point in units assuming a product selling price is $100, Fixed Costs are $8,000, Variable Costs are $20, and Operating Income is $32,000 ? 100 units 300 units 400 units 500 units
-
Given the following financial data for the Smith Corporation, calculate the length of the firm's operating cycle (OC). Sales $2,610,000 Cost of Good Sold $2,088,000 Inventory $ 278,400 Accounts...
-
The predetermined overhead rate is usually calculated Group of answer choices At the end of each year At the beginning of each month At the beginning of the year At the end of the month

Study smarter with the SolutionInn App


