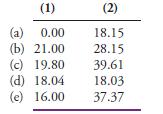
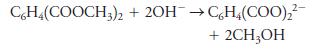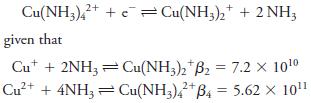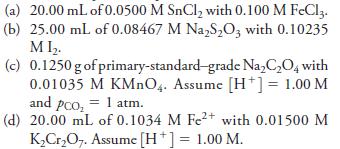Fundamentals Of Analytical Chemistry 10th Edition Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch - Solutions
Discover the ultimate resource for mastering the "Fundamentals of Analytical Chemistry, 10th Edition" by Douglas A. Skoog and others. Our comprehensive solution manual offers step-by-step answers to all textbook questions, making complex concepts easy to understand. Access the online answers key and chapter solutions to enhance your learning experience. Whether you're looking for solved problems or the complete solutions PDF, we've got you covered. Ideal for students seeking questions and answers, our instructor manual and test bank provide invaluable insights. Download this essential guide for free and elevate your chemistry skills today!
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()