A 0.9826-g sample containing dimethyl phthalate, C 6 H 4 (COOCH 3 ) 2 (194.19 g/mol), and
Question:
A 0.9826-g sample containing dimethyl phthalate, C6H4(COOCH3)2 (194.19 g/mol), and unreactive species was refluxed with 50.00 mL of 0.1104 M NaOH to hydrolyze the ester groups (this process is called saponification).
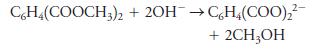
After the reaction was complete, the excess NaOH was back-titrated with 23.33 mL of 0.1597 M HCl. Calculate the percentage of dimethyl phthalate in the sample.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:





