Consider the following oxidation/reduction reactions: (a) Write each net process in terms of two balanced half-reactions. (b)
Question:
Consider the following oxidation/reduction reactions:
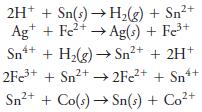
(a) Write each net process in terms of two balanced half-reactions.
(b) Express each half-reaction as a reduction.
(c) Arrange the half-reactions in (b) in order of decreasing effectiveness as electron acceptors.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:





