At a potential of -1.0 V (versus SCE), CCl 4 in methanol is reduced to CHCl 3
Question:
At a potential of -1.0 V (versus SCE), CCl4 in methanol is reduced to CHCl3 at a mercury cathode:
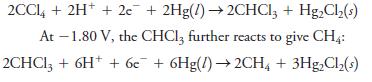
Several different 0.750-g samples containing CCl4, CHCl3, and inert organic species were dissolved in methanol and electrolyzed at -1.0 V until the current approached zero. A coulometer indicated the charge required to complete the reaction as given in the middle column of the following table. The potential of the cathode was then adjusted to -1.8 V. The additional charge given in the last column of the table was required at this potential.
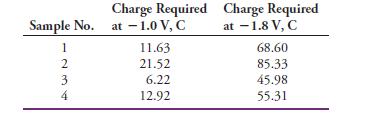
Calculate the percentage of CCl4 and CHCl3 in each mixture.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:





