The equilibrium constant for the conjugate acid-base pair is 8.00 x 10 -5 . From the additional
Question:
The equilibrium constant for the conjugate acid-base pair
![]()
is 8.00 x 10-5. From the additional information
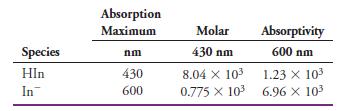
(a) Calculate the absorbance at 430 nm and 600 nm for the following indicator concentrations: 3.00 x 10-4 M, 2.00 x 10-4 M, 1.00 x 10-4 M, 0.500 x 10-4 M, and 0.250 x 10-4 M.
(b) Plot absorbance as a function of indicator concentration.
Transcribed Image Text:
HIn + H₂O = H₂0* + In¯
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
To calculate the absorbance at 430 nm and 600 nm for the different indicator concentrations we need ...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:
Students also viewed these Sciences questions
-
The equilibrium constant for the following reaction is 1.0 Ã 10-3: Cr'. (aq) + H2EDTA2-(aq)--CrEDT A-(aq) + 2H' (aq) CH2-CO2 02GH CH EDTA N-CH,--CH2- O2C-CH2 CHy-CO2-...
-
At 2000 oC the equilibrium constant for the reaction Is Kc = 2.4 Ã 103. If the initial concentration of NO is 0.175 M, what are the equilibrium concentrations of NO, N2, and O2?
-
As shown in Table 15.2, the equilibrium constant for the reaction (a) What are the masses of N2 and H2 in the equilibrium mixture? (b) What was the initial mass of ammonia placed in the vessel? (c)...
-
Prove that the function x2 x - 1 | f (x) = x 1)(x 2) is differentiable for all r E (-0, 1) U (1, 2) U (2, ). - |
-
The reinvestment of capital gains and dividends can make a significant difference in your total return. Consider the following situation to determine the difference reinvestment can make over a...
-
An Associated Press article by Eileen Alt Powell discussed the changes that banks are making to the way they calculate the minimum payment due on credit card balances. The changes are being pushed by...
-
Compute the amount that can be borrowed under each of the following circumstances: 1 A promise to repay $90,000 seven years from now at an interest rate of 6%. 2 An agreement made on February 1,...
-
A cast-iron piston ring has a mean diameter of 81 mm, a radial height h = 6 mm, and a thickness b = 4 mm. The ring is assembled using an expansion tool that separates the split ends a distance...
-
please show the calculation!! Question 1 ABC Lad started business on January | 2014, and its financial year end on December 31 cach year. It had th following Machinery as at December, 2018 5 2014...
-
Would you expect the Law of One Price to apply in the case of the following products and, if not, why not? a. A barrel of crude oil b. A litre of petrol c. A Real Madrid football club shirt d. A...
-
Three large proteins are ionized at the pH at which an electrical FFF separation is carried out. If the ions are designated A 2+ , B + , and C 3+ , predict the order of elution.
-
In Exercises find the limit graphically. Use the Sandwich Theorem to confirm your answer. lim x sin x
-
The following cost graphs illustrate various types of cost behavior: For each of the following costs, identify the cost graph that best illustrates its cost behavior as the number of units produced...
-
You are the manager of internal audit of Coverit Corporation, a large insurance company. One day you receive an urgent letter from the controller expressing his concerns about some organizational...
-
Daintree Ltd. is a large retailer that operates department stores in all major cities throughout Australia. Recently it has expanded its operations into Southeast Asia. Although each store operates...
-
Draw two points P and Q. Then sketch PQ. Add a point R on the ray so that Q is between P and R. C D A B FL E
-
Hypothesis testing and testing claims with confidence intervals are two different approaches that lead to the same conclusion. In the following activities, you will compare and contrast those two...
-
The following system of periodic tasks is scheduled and executed according to a cyclic schedule. Draw an execution trace (timeline) showing two occurances of each task. Ti ei Pi 1 8 T2 4 15 T3 3 20...
-
Can the earned income credit 1x? characterized as a form of negative income 1ax? Why or why not?
-
A summary of changes in Pen Corporation's Investment in Sam account from January 1, 2011, to December 31, 2013, follows (in thousands): ADDITIONAL INFORMATION 1. Pen acquired its 80 percent interest...
-
Bromonium ions can be captured by nucleophiles other than water. Predict the products of each of the following reactions: a.
-
For each of the following objects determine whether or not it possesses a plane of symmetry: a. b. c. d. e. f.
-
Each of the following reactions proceeds via an S N 1 mechanism and will have anywhere from two to five steps, as discussed in Section 7.6. Determine the number of steps for each reaction, and then...
-
Oct. 31: Paid salaries, $45,000 ( 75% selling, 25% administrtive). Data table Data table them to retail stores. The company has three inventory items: and floor lamps. RLC uses a perpetual inventory...
-
question 1- You borrow a simple loan of SR 500,000, interest rate is 20%, it matures in one year. what's the yied to maturity? question 2- calculate_i for One-Year Discount Bond with price(p) =...
-
Taste of Muscat is a reputed chain of restaurants operating in Oman. Assume You are working as a management accountant for this restaurant chain which is specialized in all types of Arabic food. Your...

Study smarter with the SolutionInn App


