Bromonium ions can be captured by nucleophiles other than water. Predict the products of each of the
Question:
a.
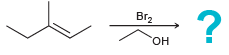
b.
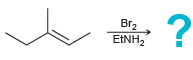
Transcribed Image Text:
Br2 "он Br2 EINH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
a b ...View the full answer

Answered By

Mamba Dedan
I am a computer scientist specializing in database management, OS, networking, and software development. I have a knack for database work, Operating systems, networking, and programming, I can give you the best solution on this without any hesitation. I have a knack in software development with key skills in UML diagrams, storyboarding, code development, software testing and implementation on several platforms.
4.90+
97+ Reviews
194+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the products of each of the following reactions. (a) (b) (c) (d) OH Cl pyridine OH (1) NaH (2) CH2l HBr OH HNOg, H2SO4 H3C
-
Predict the products of each of the following reactions: a. b. c. d. e. f. g. h. i. 1) BH3 THF 2) H2O2, NaOH Pt
-
Under acid-catalyzed conditions, epoxides can be opened by a variety of nucleophiles other than water, such as alcohols. In such a case, the nucleophile will generally attack at the more substituted...
-
8) Implement a class UserNames which requires an ArrayList of names (user names), consider the following static methods methods: initilizeUserName(), deleteUserName(), addUserName() and display...
-
What is lordosis and how does it relate to a lumbar pad?
-
Explain what the ex-dividend date is and the implications for an investor who purchases shares on that date.
-
Congress voting on womens issues. The American Economic Review (Mar. 2008) published research on how the gender mix of a U.S. legislators children can influence the legislators votes in Congress....
-
A company has total assets of $2.5 million, total liabilities of $1.8 million, and $200,000 worth of 8 percent preferred stock outstanding. What is the firms total book value? What would its book...
-
You write a call option on Gilead Sciences (GILD) with a premium of $2.74 and an exercise price of $55.01 If the share price is $57.91 when the option is exercised or expires. How much profit or loss...
-
First National Bank of Conway is considering installing two ATMs in its Southside branch. The new machines are expected to cost $37,000 apiece. Installation costs will amount to about $15,000 per...
-
Draw the mechanism of the following reaction: ONa + NaBr Br
-
For each of the following objects determine whether or not it possesses a plane of symmetry: a. b. c. d. e. f.
-
Evaluate each expression. 8 -1 - 3 -1
-
Create your own privacy philosophy. This should cover the policies you will use for email, texting, social media, and internet usage. Consider what information is being collected anout you in each...
-
1. What future markets might be attractive to Carrefour and which mode of operation would be preferable? How important is the theoretical concept of psychological distance? 2. Corporate...
-
Briefly restate your problem space and methodology. Considering your problem space and methodology, what factors are you considering in deciding whether to use a theoretical foundation or a...
-
In light of your personal experience, what strategies or approaches do you believe could be effective in creating a workplace environment where employees from diverse cultural backgrounds feel both...
-
1. Entrepreneurs hold many common traits, identify five common traits of an entrepreneur that resonate with you and discuss each one of the five traits and why they matter to you. 2. Why is...
-
Solve. |2x - 5 9
-
The manager of a local convenience store is expanding his line of small toy items. To price these new items, the manager is looking at the prices being charged by competing retailers in his area. For...
-
Draw all the isomers for each of these formulas. The total number for each is given in parentheses. (a) C3H8O (3) (b) C4H9Cl (4) (c) C4H8 (5) (d) C7H16 (9)
-
The formula C4H8O has many isomers. (a) Draw three isomers that have a carbon oxygen double bond. What functional group is present in each of them? (b) Draw three alcohols with this formula. (c) Draw...
-
Four of the ten isomers of C5H10, are shown in Figure 2.5. Draw four other isomers with this formula. Line structure Condensed Skeletal Molecular o Kekule structure model structure structure ---...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


