Calculate the theoretical potential of the following cells. Indicate whether the reaction will proceed spontaneously in the
Question:
Calculate the theoretical potential of the following cells. Indicate whether the reaction will proceed spontaneously in the direction considered (oxidation on the left; reduction on the right) or whether an external voltage source is needed to force this reaction to occur.
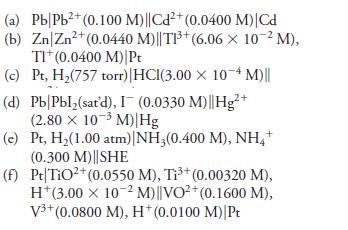
Transcribed Image Text:
(a) Pb/Pb²+ (0.100 M)||Cd²+ (0.0400 M) |Cd (b) Zn/Zn²+ (0.0440 M)||TP³ (6.06 x 10-² M), TI* (0.0400 M) Pt (c) Pt, H₂(757 torr) | HCl(3.00 x 10-4 M)|| (d) Pb/Pbl₂(sat'd), I (0.0330 M)||Hg²+ (2.80 x 10-³ M) Hg (c) (f) Pt, H₂(1.00 atm) | NH3(0.400 M), NH4+ (0.300 M)||SHE Pt TiO²+ (0.0550 M), Ti³+ (0.00320 M), H*(3.00 x 10-2 M)||VO²+ (0.1600 M), V³+ (0.0800 M), H*(0.0100 M)|Pt
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
To determine the theoretical potential of a cell we need to know the standard reduction potentials o...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:
Students also viewed these Sciences questions
-
Calculate the theoretical potential at 25C needed to initiate the deposition of
-
An RLC circuit with an alternating voltage source is shown. The source voltage vs is given by vs = vmsin(dt), where d = 2fd, in which fd is the driving frequency. The amplitude of the current, I, in...
-
In each of the following indicate which reaction will occur faster. Explain your reasoning. (a) CH3CH2CH2CH2Br or CH3CH2CH2CH2I with sodium cyanide in dimethyl sulfoxide (b) 1-Chloro-2-methylbutane...
-
1. Let f(x) = 2x+6 if x < -1 3x+2 if x -1 (a) What is lim, -1- f(x)? Your answer will depend on b. (b) What is lim,-1+ f(x)? (c) For what values of b does limx-1 f(x) exist? 2. Suppose that f and g...
-
6x 8y = 16 Determine the slope and the y-intercept. Use the slope and the y-intercept to graph the equation by hand.
-
Study the Wellmeadows Hospital case study presented in Appendix B.3. (a) In what ways would a DBMS help this organization? (b) What do you think are the main objects that need to be represented in...
-
4. Suppose that f is a real-valued function of a real variable. If f is continuous at a with f (a) < M for some MER, prove that there is an open interval I containing a such that f (x) < M for all x...
-
In January 2017, Kirkland University receives a pledge of $200,000 to be used exclusively to support research in a specialized area of communication disorders. The university's fiscal year ends on...
-
Your neighbor goes to the post office once a month and picks up two checks, one for $14,800 and one for $5,800. The larger check takes five days to clear after it is deposited; the smaller one takes...
-
Choose your preferred national, regional, and local news media in each of the groups. Identify the number of articles that criticize business or an employer-related decision. Identify the number of...
-
Under what circumstance is the curve for an oxidation/ reduction titration asymmetric about the equivalence point?
-
What is unique about the condition of equilibrium in an oxidation/reduction reaction?
-
In a report, use the sample information to 1. Summarize the growth rates in Western Europe and Greater China for Nike. 2. Summarize the growth rates in Western Europe and Greater China for Adidas. 3....
-
When a supersonic airflow, \(M=1.8\), passes through a normal shockwave under sea level conditions, what are the values of the stagnation pressure before and after the normal shockwave?
-
Eastern University, located in central Canada, prides itself on providing faculty and staff with a competitive compensation package. One aspect of this package is a tuition benefit of \($4,000\) per...
-
What is the formula for calculating return on investment (ROI)?
-
Air enters a 5.5-cm-diameter adiabatic duct with inlet conditions of \(\mathrm{Ma}_{1}=2.2, T_{1}=250 \mathrm{~K}\), and \(P_{1}=60 \mathrm{kPa}\), and exits at a Mach number of...
-
At the various activity levels shown, Taylor Company incurred the following costs. Required: Identify each of these costs as fixed, variable, or mixed. Units sold 20 40 60 80 100 a. Total salary cost...
-
Now consider the political, economic, and legal systems of China versus the United Kingdom. Explain why you think one country might be more hospitable to Cervlo than the other.
-
The Ranch 888 Noodle Company sells two types of dried noodles:ramen, at $6.50 per box, and chow fun, at $7.70 per box. So farthis year, the company has sold a total of 110,096 boxes ofnoodles,...
-
Predict the products that are expected when each of the following alkenes is treated with a peroxy acid (such as MCPBA) followed by aqueous acid: a. b. c. d. e. f.
-
For each of the products shown in the following reaction, propose a mechanism that explains its formation: Br NBS, hv Br
-
Glucose (a sugar) is produced by photosynthesis and is used by cells to store energy. Draw the most stable conformation of glucose: , Glucose
-
(International Finance) Computing a Currency changes = (e1 - e0 )/ e0 where e0 = old currency value e1 = new currency value (a) If the dinar devalues against the U.S. dollar by 45%, the U.S. dollar...
-
2. Fill in the time line for the Sawing Department. Use the time line to help you compute the number of equivalent units and the cost per equivalent unit in the Sawing Department for September Show...
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...

Study smarter with the SolutionInn App


