Predict the products that are expected when each of the following alkenes is treated with a peroxy
Question:
a.
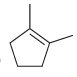
b.
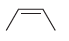
c.
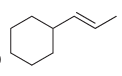
d.
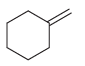
e.
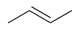
f.
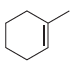
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
a b ...View the full answer

Answered By

MICHAEL K L
Let explore the education and i am having the experience. I have done the graduation in 2013
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the products that are expected when each of the following compounds is heated with concentrated HBr. a. b. c. d.
-
Draw the products that are expected when each of the following amino acids is treated with ninhydrin: (a) l-Aspartic acid (b) l-Leucine (c) l-Phenylalanine (d) l-Proline
-
When each of the following ketones is treated with aqueous sodium hydroxide, the aldol product is obtained in poor yields. In these cases, special distillation techniques are used to increase the...
-
A small company has $4,500,000 in (annual) revenue, spends 57% of its revenues on purchases, and has a net profit margin of 11.75%. They would like to increase their profits and they are looking at...
-
A current problem in the U.S. Army is the neck/shoulder fatigue experienced by helicopter pilots. To be able to fly missions at night, the pilots wear night vision goggles, which are attached to the...
-
Ben Davison and Casey Sarr discuss an important decision that will be made in a company board meeting. They know that one board member, Joan will want to keep production in China, while another board...
-
RateMyProfessors.com. A popular Web site among college students is RateMyProfessors.com (RMP). Established over 10 years ago, RMP allows students to post quantitative ratings of their instructors. In...
-
An investor sells a stock short for $36 a share. A year later, the investor covers the position at $30 a share. If the margin requirement is 60 percent, what is the percentage return earned on the...
-
Data table Complete the table below: (Round to two decimal places. Net income to three decimal places.) highlighted texts in the popup dialogue box and select Copy in order to paste its contents into...
-
Combine the following three blocks of addresses into a single block: a. 16.27.24.0/26 b. 16.27.24.64/26 c. 16.27.24.128/25
-
The following reaction is very slow. Identify the mechanism, and explain why the reaction is so slow. Br NaOH,
-
For each of the products shown in the following reaction, propose a mechanism that explains its formation: Br NBS, hv Br
-
Fill in the blank with an appropriate word, phrase, or symbol(s). To determine the number of distinct ways two or more experiments can be performed, the _________ principle can be used.
-
Gulf Shore Lawn and Garden Maintenance provides two general outdoor services: lawn maintenance and garden maintenance. The company charges customers $18.0 per hour for each type of service, but lawn...
-
Two level sections of an east highway (G=0) are to be connected. Currently, the two sections of highway are seperated by a 4000-ft (horizontal distance), 2% grade. The westernmost section of highway...
-
A solution contains 2 x 10-3 moles Ca2+/L and 3 x 10-4 moles Mg2+/L. Given the formation constants for CaEDTA2- and MgEDTA2- of 1010.6 and 108.7, respecively, calculate: 1) Concentration of MgEDTA2-...
-
The direct material (DM) price variance is $2,650 favorable and the DM usage variance is $3,000 unfavorable. The budgeted amount of DM for each unit of product is 2 lbs. to be purchased at the...
-
On January 1, 2023, AMI Corporation purchased the non-cash net assets of Oriole Ltd. for $8,399,900. Following is the statement of financial position of Oriole Ltd. from the company's year- end the...
-
Factor completely. m 6 - 1
-
10m solution. If Ka(HA) = 10 then pOH of solution will be [Given : log4=0.6] (A) 6.7 (B) Greater than 6.7 & less than 7.0 (C) Greater 7.0 & less than 7.3 (D) Greater than 7.3
-
Show the hybridization at each of the atoms, except H, in these molecules. Indicate the type of each designated bond and the orbital's that are overlapping to form it? (both) TTT a) HC=C_C_CH ...
-
What is the hybridization at all atoms, except hydrogen's, in these compounds? a) H H H H H H H H CH 4.6 c) HC-N-CH3 : : g) CHC-OH h) CHC-NHCH,
-
Draw the p orbital's that compose the conjugated part of these molecules: a) :-CH3 b) CH,=CHNH, c) H-C-C-CH=CH
-
Management makes many judgements and estimates in preparing accounts, some of which will have a significant effect on the reported results and financial position. Give examples of ZAIN estimates and...
-
What is the NPV of a project with an initial investment of $350,000 and annual cash inflows of $150,000 for the next 10 years? Cost of capital is 13% A $436,721.21 B $442,901.59 C $452,932.43 D...
-
Journal DATE DESCRIPTION POST. REF. DEBIT CREDIT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Joumalize the entries for the following transactions. Refer to the Chart of Accounts for exact wording of...

Study smarter with the SolutionInn App


