Draw the p orbital's that compose the conjugated part of these molecules: a) :-CH3 b) CH,=CHNH, c)
Question:
Draw the p orbital's that compose the conjugated part of these molecules:
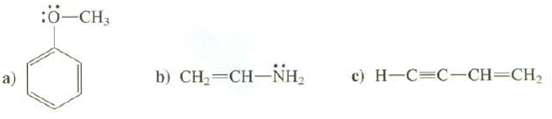
Transcribed Image Text:
a) :Ö-CH3 b) CH,=CH–NH, c) H-C-C-CH=CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
a CH3 b H 181 H c H fir 8th CH...View the full answer

Answered By

Muhammad Khurram
I have strong General Management skills to apply in your projects. Over last 3 years, I have acquired great knowledge of Accounting, Auditing, Microsoft Excel, Microsoft PowerPoint, Finance, Microsoft Project, Taxation, Strategic Management, Human Resource, Financial Planning, Business Planning, Microsoft Word, International Business, Entrepreneurship, General Management, Business Mathematics, Advertising, Marketing, Supply Chain, and E-commerce. I can guarantee professional services with accuracy.
4.80+
249+ Reviews
407+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Draw an acceptable Lewis electron dot diagram for these molecules that violate the octet rule. a. NO2 b. XeF4
-
Draw an acceptable Lewis electron dot diagram for these molecules that violate the octet rule. a. BCl3 b. ClO2
-
Draw an acceptable Lewis electron dot diagram for these molecules that violate the octet rule. a. POF3 b. ClF3
-
V = 6V 1052 3F 1-what value will be the voltage across the capacitor after 2 times constant? 2-what value will be the voltage across the capacitor after 6 seconds? 3-when will the capacitor be fully...
-
Explain the benefits of using transfer pricing within organizations?
-
Harold Limited's condensed financial statements provide the following information: Instructions (a) Determine the following: 1. Current ratio at December 31, 2017 2. Acid-test ratio at December 31,...
-
2. In eliminating unrealized profit on intercompany sales of inventory items, should gross profit or net profit be eliminated?
-
A woman considering the purchase of a custom sound stereo system for her car looked at three different systems (A, B, and C), which varied in terms of price, sound quality, and FM reception. The...
-
In computing net present value or the profitability index, managers can deal with uncertainty by 1. Using a lower discount rate for more distant cash flows 2. Using a higher discount rate for less...
-
Complete the method named charsearch in the class definition for Listops that will search the object attribute xlist (a list of strings) and return the number of strings that begin with the input...
-
What is the hybridization at all atoms, except hydrogen's, in these compounds? a) H H H H H H H H CH 4.6 c) HC-N-CH3 : : g) CHC-OH h) CHC-NHCH,
-
Show the important resonance structures for these compounds. Use the curved arrow convention to show how the electrons are moved to create each new resonance structure. N: d) CH-C b) 0-H O-H c)...
-
Explain the complex issues related to the housing of women in jails
-
Discuss the Competitive Markets and Externalities simulations (both with and without policy interventions) . What impact do policy interventions have on the supply and demand equilibrium for a...
-
The best consultant to fix issue number one is Frederick Taylor who is credited with creating the scientific management movement (Lumen, n.d.). Since Taylor's work focused on how a process could be...
-
1. Which Pepsico products are growing faster than soft drinks (why) and by what percentage? 2. Why do the fastest growing products experience a more complex supply chain? Explain. 3. What are some of...
-
Use BLUF (Bottom Line UP Front) or Brief for answering the following questions: 1) There are a number of InfoSec frameworks / models available in industry. A. What is an InfoSec framework / model? B....
-
An introduction to organizational structure. Topics such as alternative organizational structures, the reciprocal relationship between multinational strategy and structure, and how recourses affect...
-
Explain Alderfers theory of existence, relatedness, and growth.
-
The following information is for Montreal Gloves Inc. for the year 2020: Manufacturing costs Number of gloves manufactured Beginning inventory $ 3,016,700 311,000 pairs 0 pairs Sales in 2020 were...
-
For what values of x does the series converge? 00 3 n=1 (x 3)" n
-
Explain why each of the following names is incorrect: (a) 2, 2-Dirnethyl-6-ethytheptane (b) 4-Ethyl-5, 5-dirnethylpentane (c) 3.Ethyl-4, 4-dimcthylhexane (d) 5, 5, 6-Trimcthyloctane (e)...
-
Propose structures and give IUPAC names for the following: (a) A diethyldimethyihexane (b) A (3-methylbutyl)-substituted alkane
-
Consider 2-methylbutane (isopentane) Sighting along the C2C3 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. (c)...
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


