Show the important resonance structures for these compounds. Use the curved arrow convention to show how the
Question:
Show the important resonance structures for these compounds. Use the curved arrow convention to show how the electrons are moved to create each new resonance structure.
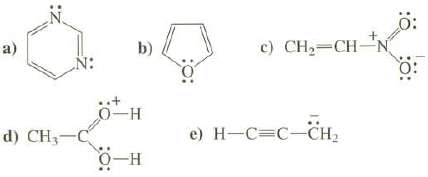
Transcribed Image Text:
N: d) CH₂-C b) 0-H O-H c) CH₂=CH-N e) H-C=C-CH₂ Ö: 0:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 46% (13 reviews)
a The unshared electron pairs on the nitrogens are not part of the conjugated pi system b One uns...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show the important resonance structures for these species. Use the curved arrow convention to show how the electrons are moved to create each new resonance structure. ) d) . CH3 H- b) CH3-N + H -H...
-
Draw the important resonance structures for these species. Use the curved arrow convention to show how the electrons are moved to create each new resonance structure. Discuss the relative...
-
Draw the important resonance structures for these species and discuss the contribution of each to the resonance hybrid. Explain whether the species has a large or a small amount of resonance...
-
You are developing an industrial building with a gross building area of 150,000 sf. The building efficiency ratio is 75%. The market gross rent is $25 psf. The vacancy rate is 5%; the cap rate is 5%;...
-
How might real-time reporting contribute to improving the competitive advantage of an organization?
-
What is a neural network? Describe two applications of neural networks.
-
2 What changes of approach and attitude would have helped the counsellor to adjust to the country?
-
PrideTalk Corp., reporting under ASPE, has provided the following information regarding its intangible assets : 1. A patent was purchased from Marvin Inc. for $1.2 million on January 1, 2013....
-
5. Resourceful Books has the following transactions in August related to merchandise inventory Click the icon to view the transactions.) Read the requirements. a. Determine the cost of goods sold and...
-
Auditors for the Internal Revenue Service (IRS) scrutinize income tax returns after they have been prescreened with the help of computer tests for normal ranges of deductions claimed by taxpayers....
-
Draw the p orbital's that compose the conjugated part of these molecules: a) :-CH3 b) CH,=CHNH, c) H-C-C-CH=CH
-
In these examples the additional structure or structures are not important contributors to the resonance hybrid for the compound represented by the first structure, explain. a) 8-8 c) CH-C=N: b) :0...
-
The IMA definition of management accounting states that: a. Management accounting is the process of gathering, reporting, and analyzing information for management decision making. b. Management...
-
How do we design an electromagnetic sensor?
-
What is a virtual breadboard?
-
Joe secured a loan of $13,000 four years ago from a bank for use toward his college expenses. The bank charges interest at the rate of 9%/year compounded monthly on his loan. Now that he has...
-
Answer these two questions 1 32 2 Number of Units Sold 3 4 ! Direct Material units per unit of production 5 i 6 Total Direct Materials Used 7! 8 Price Per Unit 9 10 Cost of Direct Materials 11 12 13...
-
Give an algorithm for converting a tree to its mirror. Mirror of a tree is another tree with left and right children of all non-leaf nodes interchanged. The trees below are mirrors to each other....
-
1 Do your studies and related activities on your course satisfy needs identified by Maslow?
-
The following information is for Montreal Gloves Inc. for the year 2020: Manufacturing costs Number of gloves manufactured Beginning inventory $ 3,016,700 311,000 pairs 0 pairs Sales in 2020 were...
-
Indicate which tests should be used. n + 1 =1 33 + 4n? + 2
-
What are the relative energies of the three possible staggered conformations around the C2C3 bond in 2, 3-dimethylbutane? (See Problem 3.42)
-
Construct a qualitative potential-energy diagram for rotation about the CC bond of 1, 2-dibromoethane. Which conformation would you expect to be more stable? Label the anti and gauche conformations...
-
Which conformation of 1, 2-dibrornoethane (Problem 3.44) would you expect to have the larger dipole moment? The observed dipole moment of 1, 2-dibromoethane is = 1.0 D. What does this tell you about...
-
September 23 for $1,050 each. On December 24 , it sold one of the diamonds that was purchased on July 9 . Using the specific identification method, its ending inventory (after the December 24 sale)...
-
Madsen Motors's bonds have 13 years remaining to maturity. Interest is paid annually, they have a $1,000 par value, the coupon interest rate is 8%, and the yield to maturity is 10%. What is the...
-
Builder Products, Incorporated, uses the weighted - average method in its process costing system. It manufactures a caulking compound that goes through three processing stages prior to completion....

Study smarter with the SolutionInn App


