Draw the important resonance structures for these species. Use the curved arrow convention to show how the
Question:
Draw the important resonance structures for these species. Use the curved arrow convention to show how the electrons are moved to create each new resonance structure. Discuss the relative contribution of each to the resonance hybrid and the overall resonance stabilization of the species.
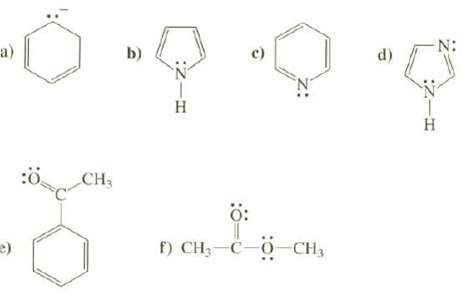
Transcribed Image Text:
a) e) :Ö CH₂ b) :ZIH H c) ő: f) CH₂-C-O-CH₂ d) :Z-H Η N:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (16 reviews)
a Each of these structures makes a similar contribution to the resonance hybrid because they have si...View the full answer

Answered By

Dominic Joseph
Education:
Bachelors' in Mechanical Engineering (2012-2016)
College - College of Engineering Trivandrum, Kerala, India
CGPA - 8.03/10
Graduated with first class distinction in 2016
Tutoring experience:
Expert Tutor (2019 - Present)
I have significant experience in tutoring multiple high school / college / post graduate students and working professionals in several fields, through multiple tutoring platforms
Subjects Taught:
• Algebra, Number systems and Complex Numbers
• Linear Algebra
• Calculus and Multivariable calculus
• Differential Equations (ODE and PDE)
• Statistics and Probability
• Data Science
• R programming, Python Programming, SAS programming
Tutored several Highschool/College students offline in the field of mathematics and statistics
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Draw the important resonance structures for these species and discuss the contribution of each to the resonance hybrid. Explain whether the species has a large or a small amount of resonance...
-
Show the important resonance structures for these species. Use the curved arrow convention to show how the electrons are moved to create each new resonance structure. ) d) . CH3 H- b) CH3-N + H -H...
-
Show the important resonance structures for these compounds. Use the curved arrow convention to show how the electrons are moved to create each new resonance structure. N: d) CH-C b) 0-H O-H c)...
-
Necked Amber purchased a bond for $1,038.90 exactly two years ago. At that time, the bond had a maturity of five years and a coupon rate of 10% (paid semi-annually). Assuming the rates below are the...
-
Indicate the type of responsibility centre that is most appropriate for each of the following: 1. A cinema in a company that operates a chain of theatres. 2. A television station owned by a large...
-
Procter & Gamble (P&G) owns a large portfolio of familiar brands such as Pampers, Tide, Bounty, Folgers, Pringles, Charmin, and Crest. P&G operates in more than 80 countries worldwide, with net sales...
-
Boating safety. Data on accidents in recreational boating in the Statistical Abstract of the United States show that the number of deaths has dropped from 1360 in 1980 to 676 in 2004. However, the...
-
Clarke, Inc., manufactures door panels. Suppose Clarke is considering spending the following amounts on a new total quality management (TQM) program: Clarke expects the new program would save costs...
-
Dash Company adopted a standard costing system several years ago. The standard costs for the prime costs (i.e., direct materials and direct labor) of its single product are: Material (6 kilograms...
-
While walking down a dark alley, Ben Bit diddle encounters a two input gate with the transfer function shown in Figure 1.48. The inputs are A and B and the output is Y. A Figure 1.48 Two-Input DC...
-
Discuss the actual structure and the amount of resonance stabilization for the examples shown in problem 3.14.
-
Show energy level diagram for the MOs of these compounds? a) H-C=N: b) H 0: C CH3 ) CHNH,
-
On August 9, 2002, Microsoft stock was selling for $48.58 per share. A $35 European call option, expiring on January 17, 2003, was selling for $13.85. Use this information to estimate the implied...
-
speed of the three phase motor does not vary greatly from the experiment. 1. Draw the symbol for a Three Phase Electric Motor. (Hint: remember the symbol table from the beginning of the semester?) 2....
-
Lifetime Insurance Company has two supporting departments (actuarial and premium), and two production departments (advertising and sales). Data from operations for the current year are as follows:...
-
Consider a wireless local area network (LAN) with an access point and 10 stations (Station 1, Station 2, Station 3, , and Station 10). Distributed coordination function (DCF), which is based on...
-
A worker needs to pump water from a reservoir to a big container that is open to the atmosphere. The water velocity at the surface of the reservoir is 2.5 m/s. The worker uses a 35-m long, 18-cm...
-
Identify each fringe benefit provided to Maggie and determine whether an exemption applies. (6 marks) Question 2: Explain the impact the fringe benefits will have on Maggie's taxable income and/or...
-
1 Describe what the organisation expected of you.
-
Use the following data to answer the next two (2) questions: Product 1 Product 2 Product 3 Direct Material Cost $25,000 $30,000 $35,000 Direct Labor Cost $30,000 $40,000 $50,000 Direct Labor Hours...
-
Find a power series representation of x 3 /(x + 2).
-
Draw structures that meet the following description (there are many possibilities): (a) Three isomers with the formula C8H18 (b) Three isomers with the formula C4H8O2
-
Draw structures of the nine isomers of C7H16.
-
In each of the following sets, which structures represent the same compounds and which represent different compounds? CH Br C (a) " , n CHH CH3CHCHCH3 Br Br CH . (b) , " (c) CH CH2CH3 CH H,H,, ,...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


