In each of the following sets, which structures represent the same compounds and which represent different compounds?
Question:
In each of the following sets, which structures represent the same compounds and which represent different compounds?
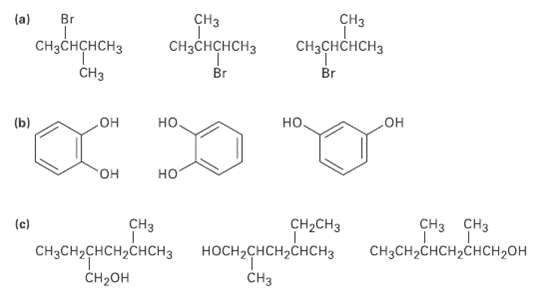
Transcribed Image Text:
CHз Br CНз (a) "одрором оидено, онрn снаснснсна CнзснсHсHз CH3CHCHCH3 Br Br CHз но. (b) но, но но но "он (c) CHз CH2CH3 CHз снз сH,сH,снсн, снсн,он НОCH-CHCH2CНCH3 CHзCH2CHсH,CНСH3 CнзCH-CHCH2CHCH2ОH CH-он CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (18 reviews)
a b Br CH3 same OH OH same CH3 H CHCHCH3 Br HO same same CH3 ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which member in each of the following sets has higher priority? (a) H or Br (b) C1 or Br (c) CH 3 or CH 2 CH 3 (d) NH 2 or OH (e) CH 2 OH or CH 3 (f) CH 2 OH or CH = O
-
Rank the compounds in each of the following sets in order of their expected reactivity toward nucleophilic acylsubstitution: (a) , , , CH, HH. NH2 (b) CHC -CCI3, CHCICF3/2
-
Arrange the compounds in each of the following sets in order of decreasing pKa, highest first. Explain your reasoning. (a) CLCH2CH2SH CH3CH2OH CH3CH2SH (b) CH,CH,OH (CH3),N-CH-CH,OH (CH3)N OH
-
Modify the symbol-table API to handle values with duplicate keys by having get() return an iterable for the values having a given key. Implement BST and Index as dictated by this API. Discuss the...
-
Janine is 25 and has a good job at a biotechnology company. She currently has $5,000 in an IRA, an important part of her retirement nest egg. She believes her IRA will grow at an annual rate of 8...
-
The associate dean of the college of business at Poly has succumbed to faculty pressure to purchase a new fax machine, although she has always contended that the machine would have minimal use. She...
-
How does a general partnership differ from a limited partnership? AppendixLO1
-
Willard Motors, Inc., employs 20 sales personnel to market its line of luxury automobiles. The average car sells for $65,000, and a 6 percent commission is paid to the salesperson. Willard Motors is...
-
think of that statement, and assumptions that are commonly used in the valuation process for an equity instrument (such as preferred or common stock) when answering the following question: Critique...
-
Barbara Ripley and Fred Nichols decide to organize the ALL-Star partnership. Ripley invests $15,000 cash and Nichols contributes $10,000 cash and equipment with a cost of $7,000 and accumulated...
-
Draw structures of the nine isomers of C7H16.
-
There are seven constitutional isomers with the formula C4H10O. Draw as many as you can.
-
a b Quantity A is greater. Quantity B is greater. The two quantities are equal. The relationship cannot be determined from the information given. Quantity A 6a + 12ab + 6b a+b Quantity B 6(a + b)
-
How do socio-cognitive mechanisms, such as social identity theory and self-categorization theory, contribute to the formation and maintenance of organizational culture ?
-
How do you Sales Forecast and an Expense forecast for future years?
-
2. Do you really think the Bono case described in Ch. 2 is a genuine ethical conflict? Explain. 6. Describe the ethical issue in the Siemens case
-
How do I calculate using the SPC method if my key metric is time
-
Labor Standards: Where Do They Belong on the International Trade Agenda? Author(s): Drusilla K. Brown Link. https://viu.summon.serialssolutions.com/?#!/search?....
-
apply the knowledge, skills and understanding gained to your own research project. LO7
-
The process of collaborative goal setting by a manager and subordinate, the extent to which goals are accomplished is a major factor in evaluating and rewarding the subordinate's performance. It is...
-
Tungsten has a body-centered cubic crystal structure. Using a metallic radius of 139 pm for the W atom, calculate the density of tungsten.
-
Deduce the structures of compounds A, B, and C, which all have the formula C6H10. As you read the information that follows, draw reaction flowcharts (roadmaps) like those in Problems 8.24 and 8.52....
-
Ricinoleic acid, a compound that can be isolated from castor oil, has the structure CH3(CH2)5CHOHCH2CH==CH(CH2)7CO2H. (a) How many stereoisomers of this structure are possible? (b) Write these...
-
There are two dicarboxylic acids with the general formula HO2CCH==CHCO2H. One dicarboxylic acid is called maleic acid; the other is called fumaric acid. When treated with OsO4, followed by...
-
A family has a $117,443, 25-year mortgage at 5.4% compounded monthly. (A) Find the monthly payment and the total interest paid. (B) Suppose the family decides to add an extra $100 to its mortgage...
-
Comparing the actual and planned cost of a consulting engagement completed by an engineering firm such as Allied Engineering.
-
What is the NPV of a project that costs $34,000 today and is expected to generate annual cash inflows of $11,000 for the next 7 years, followed by a final inflow of $14,000 in year 8. Cost of capital...

Study smarter with the SolutionInn App


