Rank the compounds in each of the following sets in order of their expected reactivity toward nucleophilic
Question:
Rank the compounds in each of the following sets in order of their expected reactivity toward nucleophilic acylsubstitution:
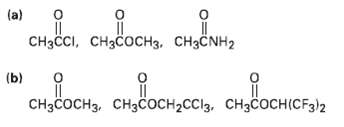
Transcribed Image Text:
(a) сира, сбсост, сндит, CHзссі, сHзсосHз. сНзсNH2 (b) CHзCоснз снзсосн-CCI3, CHзCоснICF3/2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
a Most reactive CH3CCI Least reactive CH3COCH3 ...View the full answer

Answered By

Mary Njunu
I posses Vast, diversified knowledge and excellent grammar as a result of working in ACADEMIC WRITING for more than 5 years. I deliver work in various disciplines with assurance of quality work. I purpose at meeting the clients’ expectations precisely. Let’s work together for the best and phenomenal grades.
4.90+
929+ Reviews
2557+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Rank the compounds in each of the following groups in order of decreasing acidity: (a) Acetic acid, ethane, ethanol (b) Benzene, benzoic acid, benzyl alcohol (c) Propanedial, 1, 3-propanediol,...
-
Rank the compounds within each of the following sets in order of increasing basicity, and explain your reasoning. (a) Pyridine, 3 - nitropyridine, 3 - chlorogyridine (b) imidazole and thiazole
-
Arrange the compounds in each of the following sets in order of decreasing pKa, highest first. Explain your reasoning. (a) CLCH2CH2SH CH3CH2OH CH3CH2SH (b) CH,CH,OH (CH3),N-CH-CH,OH (CH3)N OH
-
The position of a particle moving along 12t2 2t, where r is in meters and t is in an r-axis is given by x = seconds. i Determine the acceleration of the particle at t = 3.0 s. ii What are the...
-
Why is it a smart strategy to thank an interviewer, to follow up, and even to send a rejection follow-up message? Are any risks associated with this strategy?
-
The financial statements for Harridges Ltd are given below for the two years ended 30 June 2009 and 2010. Harridges Limited operates a department store in the centre of a small town. Dividends were...
-
KEY TERMS Define each of the following terms: a. Mission statement; corporate scope; statement of corporate objectives; corporate strategies b. Operating plan; financial plan c. Spontaneously...
-
Suppose that the government of Brazil took possession of the cacao farms of a chocolate factory owned by a U. S. firm. What rights would the U. S. factory have? What limits exist on those rights?
-
In your own words, explain why a tax credit is more valuable than a tax deduction of the same dollar amount.
-
During 2020, Darwin Corporation started a construction job with a contract price of $4.2 million. Darwin ran into severe technical difficulties during construction but managed to complete the job in...
-
Show the mechanism of the following nucleophilic acyl substitution reaction, using curved arrows to indicate the electron flow in eachstep: Nat -H CH
-
Predict the products of the following nucleophilic acyl substitutionreactions: (b) (a) NaOH NH3 H20 H (d) (c) Na* "OCH3 C CH3NH2 SCH3 "CH
-
(a) Design the circuit in Figure P3.42 such that \(I_{D Q}=0.25 \mathrm{~mA}\) and \(V_{D}=-2 \mathrm{~V}\). The nominal transistor parameters are \(V_{T P}=-1.2 \mathrm{~V}, k_{p}^{\prime}=\) \(35...
-
Question 1 [40 marks] (a) Table 1 present experimental data related to the absorbance of two compounds over a range of concentration, in a UV-Vis cell with path length I = 1.0 cm. From this table:...
-
i. The following table presents data on wholesale gas prices for the major capital cities in the Eastern-half of Australia, from 2011-12 to 2022-23. Use this data to construct a single, time-series...
-
Problem 1 Using the same Fourier-Method approach as used in lecture, consider a beam loaded as shown below. 290 -q. Cos 280 x Shane land V-280 Distributed load w = =-80 . Cos[X] a. What are the...
-
Think about a Floor Warden training program for that company - and write me another email (attached here as a Word document) as if I were the leader of your organization to tell me about the...
-
A particle travels around the curve shown, following ? = ? 0 . 2 ? ? , ?with ? ( ? ) = 0 . 5 ? 2 rad. At the moment ? = ? , ?determine the speed and acceleration of the particle. ? = , ? ? ? = , ? ?...
-
The chief financial officer (CFO) of a publicly-owned restaurant chain notices that the bonus for the chief executive officer (CEO) is much higher than anticipated for the year. She suspects that the...
-
Find a polar equation for the curve represented by the given Cartesian equation. 4y 2 = x
-
The compound C 3 H 4 has two double bonds. Describe its bonding and geometry, using a valence bond approach.
-
Which of the ring-opening reactions given in Fig. Pl 1.48 should occur most readily? Explain.
-
Explain how you could differentiate between the compounds in each of the following pairs by using simple physical or chemical tests that give readily observable results, such as obvious solubility...
-
Explain how you could differentiate between the compounds in each of the following pairs by using simple physical or chemical tests that give readily observable results, such as obvious solubility...
-
Pottery Ranch Inc. has been manufacturing its own finials for its curtain rods. The company is currently operating at 100% of capacity, and variable manufacturing overhead is charged to production at...
-
3. How much life insurance do you need? Calculating resources - Part 2 Aa Aa E Paolo and Maria Rossi have completed Step 1 of their needs analysis worksheet and determined that they need $2,323,000...
-
On March 1, LGE asks to extend its past-due $1,200 account payable to Tyson, Tyson agrees to accept $200 cash and a 180-day, 8%, $1,000 note payable to replace the account payable. (Use 360 days a...

Study smarter with the SolutionInn App


