Predict the products of the following nucleophilic acyl substitutionreactions: (b) (a) NaOH NH3 H20 H
Question:
Predict the products of the following nucleophilic acyl substitutionreactions:
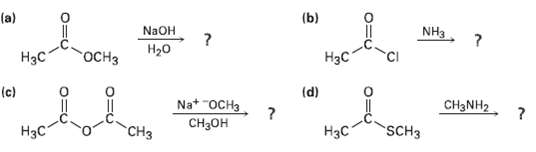
Transcribed Image Text:
(b) (a) NaOH NH3 H20 оСHз Нас Нэс (d) (c) Na* "OCH3 Cнзон CH3NH2 SCH3 Нзс "CHз НаС
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Strategy Identify the nucleophile boxed and the leavin...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show the mechanism of the following nucleophilic acyl substitution reaction, using curved arrows to indicate the electron flow in eachstep: Nat -H CH
-
Predict the products of the following reactions: (a) Excess NH3 + Ph - CH2CH2CH2Br (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) (m) (n) (o) (p) (q) (r) (I) NaN3 (2) LiAIH (3) H30 1-bromopentane CH3...
-
Predict the products of the following reactions. (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) OH CI CH NH2 Ph-C-CI+ NH2 0 + (D LiAIH (2) H20 (2) H,0 -. O (I) excess PhMgBr (2) H,o ( CH Mgl (2)...
-
In five sentences or more answer the following question: Was Japanese Internment necessary to protect Americas national security? Why / why not?
-
What can you do to appear professional when a potential employer contacts you by phone for a screening interview or to schedule a job interview?
-
A year ago Pod Limited bought 225,000 £1 fully paid ordinary shares of Pea Limited for a consideration of £500,000. Pea Limiteds share capital and share premium were each the same as at...
-
Examine the following statement: Using regression to predict items such as inventories is better than basing such predictions on last years Inventory/Sales ratio because regression helps smooth out...
-
The following graph shows the labor market for research assistants in the fictional country of Universalia. The equilibrium wage is $10 per hour, and the equilibrium number of research assistants is...
-
You are an investor who is long 1000 shares of XYZ stock. The current price is $125.94. You only want to risk $6 in the stock between now and the May expiration (21 days). You also are fine with...
-
1. Would you characterize television programming decisions as structured or unstructured? Explain. What type of decision-making condition would you consider this to be? Explain. 2. What criteria did...
-
Rank the compounds in each of the following sets in order of their expected reactivity toward nucleophilic acylsubstitution: (a) , , , CH, HH. NH2 (b) CHC -CCI3, CHCICF3/2
-
The following structure represents tetrahedral alkoxide ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the starting...
-
In Exercises, find f x (x, y) and f y (x, y). Then find f x (2, -1) and f y (-4, 3). Leave the answers in terms of e in Exercises. (x, y) = -6e 4x-3y
-
7. Chicago Corp. obtained the following information from the Raw Materials Inventory account and purchasing records for the first quarter of the current year: Beginning Raw Materials Ending Raw...
-
Suppose that i t =6% (n=1), and that future short term interest rates (n=1) for the next 3 years (starting next year) are expected to be: 4%, 2%, 2%. Suppose that the liquidity premium is zero for...
-
Mechanical Vibrations HW Use the modal analysis and numerical integration to compute and plot the time response of the system, which has the equations of motion [8 0 01 (1) 48 -12 01(x1 0 0 8 02-12...
-
Submit excel file with graph and exchange rate analysis. FOREIGN EXCHANGE RATESTHE YEN FOR DOLLARS. The Federal Reserve System Web site, www.federalreserve.gov/releases/H10/hist , provides historical...
-
Part 1: There are many types of communication styles used in the workplace. Choose what you think is your leadership style: north, south, east, or west. Click The Leadership Compass Self-Assessment...
-
State the purpose of regularly preparing a balance sheet for a hospitality business.
-
Find the velocity, acceleration, and speed of a particle with the given position function. r(t) = (t 2 , sin t - t cos t, cos t + t sin t), t > 0
-
The structure of acetylsalicylic acid (aspirin) is shown here. How many pi bonds are present in acetylsalicylic acid? How many sigma bonds? What parts of the molecule are free to rotate? What parts...
-
Explain why a mixture of two isomeric ethers is formed in the following reaction. NaBH
-
Predict the absolute configuration of the major diol product formed by treatment of (S)-2 ethyl- 2-methyloxirane with water in the presence of an acid catalyst.
-
When (3s,4S)-4-methoxy-3-methyl- I -pentene is treated with mercuric acetate in methanol solvent, then with NaBH4, two isomeric compounds with the formula C8H18O2, are isolated. One, compound A, is...
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


