The structure of acetylsalicylic acid (aspirin) is shown here. How many pi bonds are present in acetylsalicylic
Question:
The structure of acetylsalicylic acid (aspirin) is shown here. How many pi bonds are present in acetylsalicylic acid? How many sigma bonds? What parts of the molecule are free to rotate?
What parts are rigid?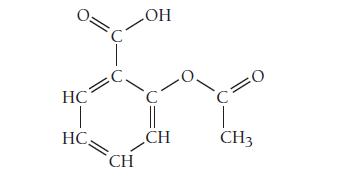
Transcribed Image Text:
HC HC C. CH OH CH CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
Pi Bonds and Sigma Bonds 1 Pi Bonds There are no pi bonds in the structure of acetylsa...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The structure of caffeine, present in coffee and many soft drinks, is shown here. How many pi bonds are present in caffeine? How many sigma bonds? Insert the lone pairs in the molecule. What kinds of...
-
How many pi bonds and sigma bonds are there in the tetracyanoethylene molecule? N=C C=N C=C C=N N=C
-
There is a tiled roof on a residential house located close to Bankstown Airport. The roof pitch is 25 and the area is 60 m2, which is draining rainwater to a single downpipe connected to a rainwater...
-
A company currently sells 8,280 basketballs (units) per year for $25 each. The company can make up to 10,280 basketballs per year. Each basketball made includes $15 In variable costs and $6.50 of...
-
The management of Wallingford MicroBrew is considering the purchase of an automated bottling machine for $80,000. The machine would replace an old piece of equipment that costs $33,000 per year to...
-
Taxes and transfers Recall that we define taxes, T, as net of transfers. In other words, \[ T=\text { Taxes }- \text { Transfer Payments } \] a. Suppose that the government increases transfer...
-
Make a detailed plan incorporating your answers for Michael to follow in dealing with his present bartenders.
-
In November 2014, after having incorporated Cookie Creations Inc., Natalie begins operations. She has decided not to pursue the offer to supply cookies to Biscuits. Instead, she will focus on...
-
Hannah Ortega is considering expanding her business. She plans to hire a salesperson to cover trade shows. Because of compensation, travel expenses, and booth rental, fixed costs for a trade show are...
-
Prevosti Farms and Sugarhouse pays its employees according to their job classification. The following employees make up Sugarhouse's staff: Employee Number Name and Address Payroll information...
-
The compound C 3 H 4 has two double bonds. Describe its bonding and geometry, using a valence bond approach.
-
The genetic code is based on four different bases with the structures shown here. Assign a geometry and hybridization to each interior atom in these four bases. a. Cytosine b. Adenine c. Thymine d....
-
Using the data in Figures 9.15 and 9.16, calculate E for the reaction Na(g) + Cl(g) Na + (g) + Cl (g). Trends in First lonization Energy Ionization energy (kJ/mol) 2500 2000. 1500- 1000- H 1312 0...
-
Carla Vista Corp. sponsors a defined benefit pension plan for its employees. On January 1, 2025, the following balances relate to this plan Plan assets $489,900 Projected benefit obligation 616,700...
-
Question 2 of 8 Shirts were purchased for $12.50 each and were marked up by $18.75. During Christmas, they were discounted by $6.85 per shirt. a. What was the rate of markdown? % Round to two decimal...
-
The cost versus quality decision is one that only few companies get right. What is the cost of quality? It is very high for some companies such as Ford and Bridgestone/Firestone, whose reputations...
-
Find the absolute maximum and absolute minimum values of the function f(x) (x-2)(x-5)+7 = on each of the indicated intervals. Enter 'NONE' for any absolute extrema that does not exist. (A) Interval =...
-
4. Roll one 10-sided die 12 times. The probability of getting exactly 4 eights in those 12 rolls is given by (a) 10 9 4 10 10 (b) HA 9 -HAA (c) 1 (d) 9 (c) 10 9 () 10
-
Draw the formula for methanol, CH3OH, and (where appropriate) indicate the bond polarity with an arrow,. (The C atom is bonded to three H atoms and the O atom.)
-
In Problems 1522, find the principal needed now to get each amount; that is, find the present value. To get $750 after 2 years at 2.5% compounded quarterly.
-
The connection is made using a bolt and nut and two washers. If the allowable bearing stress of the washers on the boards is (Ï b ) allow = 2 ksi, and the allowable tensile stress within the...
-
The lever is attached to the shaft A using a key that has a width d and length of 25 mm. If the shaft is fixed and a vertical force of 200 N is applied perpendicular to the handle, determine the...
-
If A and B are both made of wood and are 3/8 in. thick, determine to the nearest 1/4 in. the smallest dimension h of the vertical segment so that it does not fail in shear. The allowable shear stress...
-
Johnson Limited had the following information available: The amount of cash paid for insurance premiums by Johnson during 2020 was: Select one: a. $615,000. b. $485,000. c. $600,000. d. $515,000.
-
FACTORS AFFECTING INTEREST RATE ARE DISCOUNT RATE, DEFLATION AND INVESTOR EXPECTATIONS Select one: True False --------------- Please Solve As soon as Solve quickly I get you thumbs up directly...
-
Protrade Corporation acquired 80 percent of the outstanding voting stock of Seacraft Company on January 1, 2020, for $460,000 in cash and other consideration. At the acquisition date, Protrade...

Study smarter with the SolutionInn App


