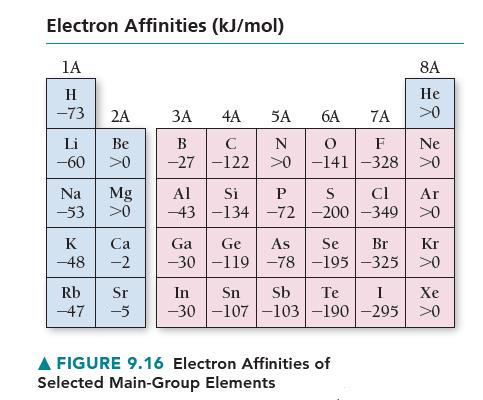
Using the data in Figures 9.15 and 9.16, calculate E for the reaction Na(g) + Cl(g)
Question:
Using the data in Figures 9.15 and 9.16, calculate ΔE for the reaction Na(g)+ Cl(g) → Na+ (g)+ Cl– (g).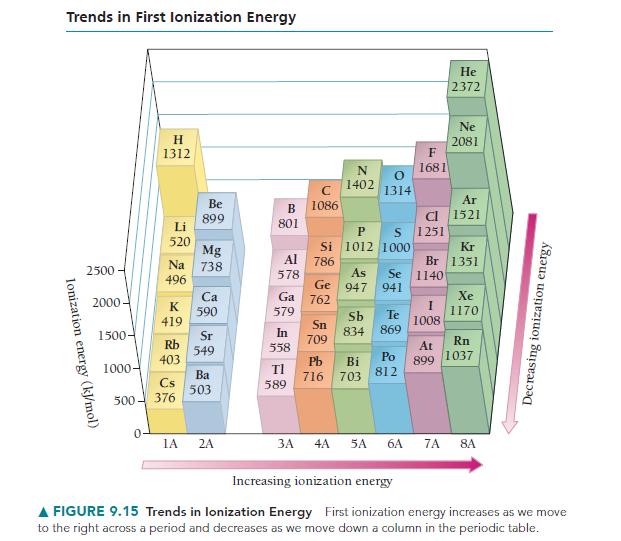
Transcribed Image Text:
Trends in First lonization Energy Ionization energy (kJ/mol) 2500 2000. 1500- 1000- H 1312 0 Li 520 Na 496 Rb 403 Cs 500-376 K 419 Be 899 Mg 738 Ca 590 Sr 549 Ba 503 1A 2A B 801 Al 578 Ga 579 In 558 ΤΙ 589 3A 1086 P Si 1012 786 Ge 762 Sn 709 N 1402 1314 Pb 716 Cl S 1251 1000 As 947 941 Sb Te 834 869 F 1681 Se 1140 Bi Po 703 812 4A 5A 6A I 1008 Kr Br 1351 At 899 He 2372 7A Ne 2081 Ar 1521 Xe 1170 Rn 1037 8A Decreasing ionization energy Increasing ionization energy A FIGURE 9.15 Trends in lonization Energy First ionization energy increases as we move to the right across a period and decreases as we move down a column in the periodic table.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
There are two main terms to consider when calculating E for the reaction Nag Clg Nag Clg th...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using the data in Table 4-3, calculate the G° for ring flip to the other conformation of the molecules depicted in Problem 30. Make sure that the sign (i.e., positive or negative) of your values...
-
Calculate the heat of vaporization, Hovap, of water, using standard enthalpies of formation (Table 6.2). 14270 8 099 0740 9 6416 3818 02015 0 or Hj H Si Si Si Si Si Si A A A A A A So Na Na Na Na Na...
-
A 6 kg block of wood resting on a platform was hit by a 0.08 kg of bullet travelling upward with a speed of 951 m/s. The bullet emerges from the block with a speed of 783 m/s. How much force was...
-
Misty Cumbie worked as a waitress at the Vita Caf in Portland, Oregon. The caf was owned and operated by Woody Woo, Inc. Woody Woo paid its servers an hourly wage that was higher than the states...
-
Willingham Company manufactures raingear. During 2010 Willingham Company decided to issue bonds at 8% interest and then used the cash to purchase a significant amount of treasury stock. The following...
-
Consider a refrigerator that operates on the vapor compression refrigeration cycle with R-134a as the working fluid. The refrigerant enters the compressor as saturated vapor at 160 kPa, and exits at...
-
How is procedural knowledge represented in the ACT-R model?
-
Creative Ideas Company has decided to introduce a new product. The new product can be manufactured by either a capital-intensive method or a labor-intensive method. The manufacturing method will not...
-
The income statements for Urban Outfits, Inc. are presented below: Urban Outfits, Inc. Income Statements Year Ended December 31 Current Year Sales Revenue $800,059 Cost of Goods Sold 398,202 Gross...
-
A compound of molecular formula C 8 H 8 O gives the IR and NMR spectra shown here. Propose a structure, and show how it is consistent with the observed absorptions. wavelength (um) 5,5 6. 8 9 10 2.5...
-
Only trace amounts of the synthetic element darmstadtium, atomic number 110, have been obtained. The element is so highly unstable that no observations of its properties have been possible. Based on...
-
A carbon atom can absorb radiation of various wavelengths with resulting changes in its electron configuration. Write orbital diagrams for the electron configuration of carbon that results from...
-
Write out the addition and multiplication tables for Zs.
-
What do you think about an 'employee-centric' rather than an 'employer-centric' PMS. Which would work better in your current (or prior) organization? Make sure to provide specific examples to justify...
-
How do I relate the below case study to RLR - Responsible Leadership for Relations? Relate and analyses in detail....
-
How do advanced integrative approaches, combining elements of cognitive-behavioral therapy, mindfulness, and somatic experiencing, offer comprehensive solutions for addressing the multifaceted nature...
-
What can you do to plan ahead and educate others about the international groups? Consider How do you communicate during the meeting with your colleagues?
-
What do you think should be the role of personality tests in candidate selection? Do you think they should play a major, minor or no part in an organization\'s hiring decision for a job? What are...
-
Why would management be interested in the revision of probabilities?
-
One study found that the elderly who do not have children dissave at about the same rate as the elderly who do have children. What might this finding imply about the reason the elderly do not dissave...
-
Consider the couple Ox + e Red with the oxidized and redu ced species at unit activity. What must be the value of E for this half-cell if the reductant Red is to liberate hydrogen at 1 atm from a....
-
By finding appropriate half-cell reactions, calculate the equilibrium constant at 298.15 K for the following reactions: a. 4NiOOH(s) + 2 2 O(l) 4Ni(OH) 2 (s) + O 2 (g) b. 4NO 3 (aq)+ 4H + (aq)...
-
The cell potential E for the cell Pt(s)|H 2 (g, a H2 = 1) H + (aq, a H+ = 1)NaCl(aq, m = 0.300) AgCl(s) Ag(s) is +0.260 V. Determine Cl assuming that = Na+ = Cl .
-
Which one of the following is the primary source of a firm's value? A. Common stock value increases B. Operating cash flows C. Cash from investing and financing sources D. Net income
-
9 of 10. For a return filed in 2023, what is the maximum penalty the IRS can assess against a paid tax return preparer who fails to satisfy the due diligence requirements when preparing a return for...
-
Critical Thinking Problem 3 . 2 ( Algo ) Sole Proprietorship LO 3 - 1 , 3 - 2 , 3 - 3 , 3 - 4 , 3 - 5 , 3 - 6 Sara - Jayne Parsons is an architect who operates her own business. The accounts and...

Study smarter with the SolutionInn App


