A compound of molecular formula C 8 H 8 O gives the IR and NMR spectra shown
Question:
A compound of molecular formula C8H8O gives the IR and NMR spectra shown here. Propose a structure, and show how it is consistent with the observed absorptions.
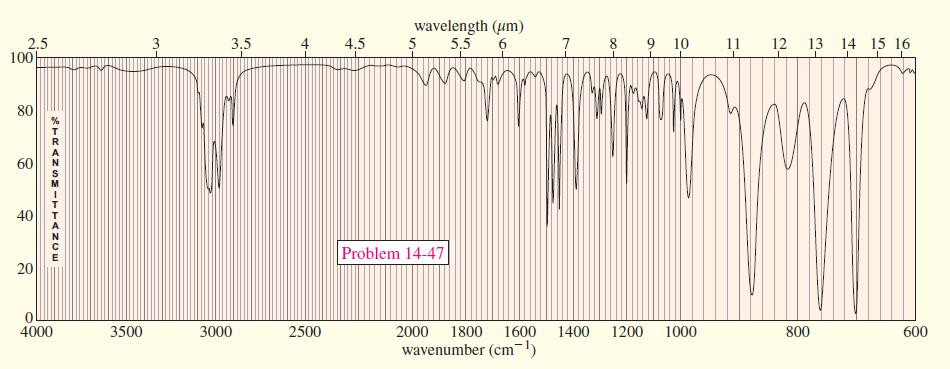
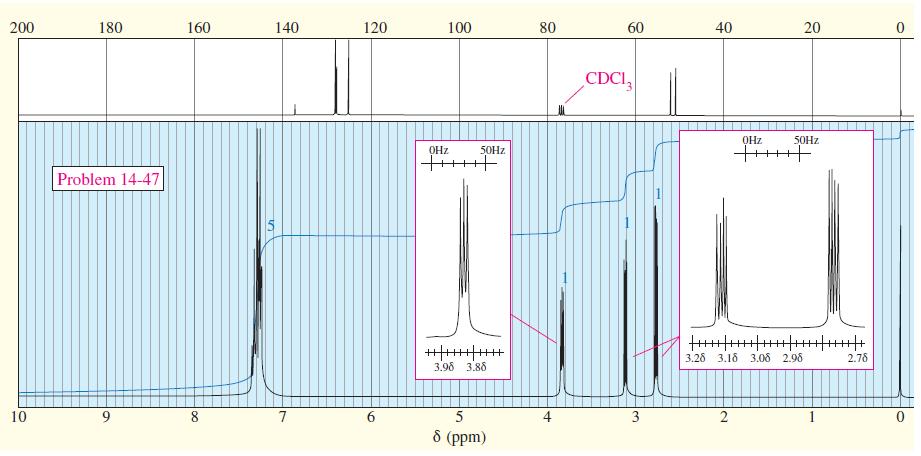
Transcribed Image Text:
wavelength (um) 5,5 6. 8 9 10 2.5 100 3.5 4.5 5 11 12 13 14 15 16 80 60 40 Problem 14-47 4000 3500 3000 2500 2000 1800 1600 1400 1200 1000 800 600 wavenumber (cm) 7- TRANGMITTANCE 20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (17 reviews)
A compound of molecular formula C 8 H 8 O gives the IR and NMR spect...View the full answer

Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An unknown compound of molecular formula C5H9NO gives the IR and NMR spectra shown here. The broad NMR peak at δ7.55 disappears when the sample is shaken with D2O. Propose a structure,...
-
An unknown compound gives the NMR, IR, and mass spectra shown next. Propose a structure, and show how it is consistent with the observed absorptions. Show fragmentations that account for the...
-
An unknown compound gives the following mass, IR, and NMR spectra. Propose a structure, and show how it is consistent with the spectra. Show the fragmentations that give the prominent peaks at m/z...
-
A health club has cost and revenue functions given by C = 10,000 + 35q and R = pq, where q is the number of annual club members and p is the price of a one year membership. The demand function for...
-
Find the activity coefficient of H+ in a solution containing 0.010 M HCl plus 0.040 M KClO4. What is the pH of the solution?
-
The physicians in Problem 3-36 have been approached by a market research firm that offers to perform a study of the market at a fee of $5,000. The market researchers claim their experience enables...
-
Explain with the help of a chart the various elements of cost.
-
Caustic Chemicals management identified the following cash flows as significant in their year end meeting with analysts: During the year Caustic repaid existing debt of $312,080 and raised additional...
-
Ravi is an experienced mechanic who has worked for many years at major automotive dealerships and garages servicing a wide range of vehicles and has been certified by multiple automotive brands in...
-
a. Ash decides to allocate $4 million to fund the exhibit. Given the pieces available and the specific requirements from Ash and Celeste, formulate and solve a binary integer programming problem to...
-
An acid-catalyzed reaction was carried out using methyl cellosolve (2-methoxyethanol) as the solvent. When the 2-methoxyethanol was redistilled, a higher-boiling fraction (bp 162 C) was also...
-
A new graduate student was studying the insecticidal properties of a series of polycyclic epoxides. He epoxidized alkene A using two different methods. First he used MCPBA, which gave an excellent...
-
Platteville Eye Clinic is considering investing in new optical scanning equipment. It has two options: Option A would have an initial lower cost but would require a significant expenditure for...
-
Analysis of workforce data, performance, and engagement. Datasets: Employees Table Column Name Data Type Description employee_id Integer Unique identifier for each employee department_id Integer...
-
Discuss your observations of the Data Wrangling process. Does this exercise highlight why data wrangling and preparation can take up 60-70% of the total data analysis process? it does. How do i say...
-
Examine potential implications od regulations, legislation and standards upon decision making in a hospitality organisation, providing specific examples
-
54. .. A baton twirler in a marching band complains that her baton is defective (Figure 9-48). The manufacturer specifies that the baton should have an overall length of L = 60.0cm and a total mass...
-
New United Motor Manufacturing, Inc. was an American automobile manufacturing company in Fremont, California , jointly owned by General Motors and Toyota that opened in 1 9 8 4 and closed in 2 0 1 0...
-
The mass m of a raindrop increases as it picks up moisture during its vertical descent through still air. If the air resistance to motion of the drop is R and its downward velocity is v, write the...
-
Given find the value of k. es 1 e kx dx = 1 4'
-
Using resonance arguments, state which ion or radical within each set is more stable. Explain. CHj HC-C CH2 or HC CH CH CH2
-
Use a Frost circle to determine the -electron structure of the cyclopropenyl cation, which has two electrons cyclopropenyl cation
-
Do you think it would be possible to have an aromatic free radical? Why or why not?
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


