What is the hybridization at all atoms, except hydrogen's, in these compounds? a) H H H H
Question:
What is the hybridization at all atoms, except hydrogen's, in these compounds?
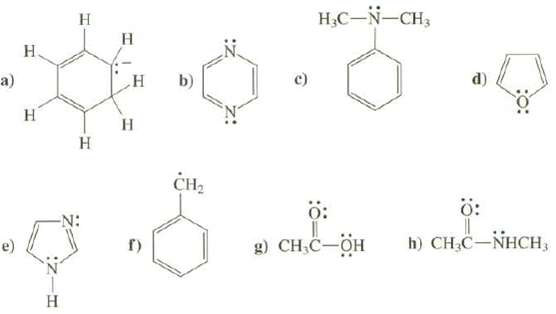
Transcribed Image Text:
a) H H H H H H H H CH₂ 4.6 c) H₂C-N-CH3 Ö: Ö: g) CH₂C-OH h) CH₂C-NHCH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
H sp2 sp2 H H sp2 Sp2 H H sp2 H s...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
In the hydrocarbon (a) What is the hybridization at each carbon atom in the molecule? (b) How many Ï bonds are there in the molecule? (c) How many Ï bonds? (d) Identify all the 120o bond...
-
What is the hybridization at the indicated atoms in these compounds? a) CHCH=CH_NHCH3 12 3 c) CH,=CH0CH, 1 2 3 4 5 0: b) CHC0CH, 1 d) 2 1 NH
-
What is the hybridization of the indicated atom in each of the following molecules? a. b. c. d. e. f. CH CH CH CH CCH CH3CH2OH CH3CH NCH3 CH:OCH CH
-
In the proposal, the contractor estimated that 50 units of a specialty part are required. Each unit costs $300. There is a minimum buy requirement of 100 units. You contact the vendor and confirm the...
-
Explain how negotiation between the supplying and buying units may be used to set transfer prices. How does this relate to the general transfer pricing rule?
-
Jupiter Corp. provides at no extra charge a two-year warranty with one of its products, which was first sold in 2017. In that year, Jupiter sold products for $2.5 million and spent $68,000 servicing...
-
1. Why are intercompany transactions between a parent and its subsidiary eliminated in preparing a consolidated financial statement?
-
1. Notwithstanding the law as applied, do you believe an employer should be able to change the terms of pension plan qualifications once individuals have begun to avail themselves of the benefits?...
-
A bullet structure Floating rate bond has a DM of 2.14%. The quoted margin is 1.40% while the current benchmark rate is equal to 0.50%. Given this information, what is the price of the bond per $100...
-
On July 1, 2016, Pat Glenn established Half Moon Realty. Pat completed the following transactions during the month of July: a. Opened a business bank account with a deposit of $25,000 from personal...
-
Show the hybridization at each of the atoms, except H, in these molecules. Indicate the type of each designated bond and the orbital's that are overlapping to form it? (both) TTT a) HC=C_C_CH ...
-
Draw the p orbital's that compose the conjugated part of these molecules: a) :-CH3 b) CH,=CHNH, c) H-C-C-CH=CH
-
What should you check before applying Theorem 10.13 to find the area of the region bounded by the graph of r = f ()? THEOREM 10.13 Area in Polar Coordinates If f is continuous and nonnegative on the...
-
Carol's Cupcakes has grown from a home business into a one of the largest event and wedding catering companies in the area. Its founder, Carol Thompson, first dreamed of owning her own company while...
-
Many things have changed for businesses in 2022. The previous 2 business years of 2020 and 2021 have tested businesses and the workforce like nothing else. Not only were profits reduced, and...
-
1) Virginia Tech's motto is "Ut Prosim" which means 'That I May Serve'. Share how you contribute to a community that is important to you. How long have you been involved? What have you learned and...
-
Person Is Arianna Grande Answer all questions Who are they? How successful are they? Why would companies be interested in partnering with them? Identify one company from their industry that you feel...
-
Imagine you have just retired after a long and very successful career (as a physiotherapist). Congratulations! You've made such an impact in the world that business and community leaders from around...
-
Describe the difference between Herzbergs hygiene factors and motivational factors.
-
Assume a simple Keynesian depression economy with a multiplier of 4 and an initial equilibrium income of $3,000. Saving and investment equal $400, and assume full employment income is $4,000. a. What...
-
Calculate lim 11 0 In n n
-
For each of the following compounds, draw an isomer that has the same functional groups. Each intersection of lines represents a carbon atom with the appropriate number of hydrogensattached. ( CH (a)...
-
Give IUPAC names for the followingcompounds: H CHCH2CCH H CH CHH2CH2CH (b) (e) CHH2H2C CH (a) H-H H CH3CH2CHCH2CH,CHCH3 (e) CH H CHCH2CH2CHCH2CH CH CH-H>H3 (d)
-
Name the five isomers of C6H14. Discuss.
-
Your company BMG Inc. has to liquidate some equipment that is being replaced. The originally cost of the equipment is $120,000. The firm has deprecated 65% of the original cost. The salvage value of...
-
1. What are the steps that the company has to do in time of merger transaction? And What are the obstacle that may lead to merger failure? 2.What are the Exceptions to not to consolidate the...
-
Problem 12-22 Net Present Value Analysis [LO12-2] The Sweetwater Candy Company would like to buy a new machine that would automatically "dip" chocolates. The dipping operation currently is done...

Study smarter with the SolutionInn App


