For each of the following compounds, draw an isomer that has the same functional groups. Each intersection
Question:
For each of the following compounds, draw an isomer that has the same functional groups. Each intersection of lines represents a carbon atom with the appropriate number of hydrogensattached.
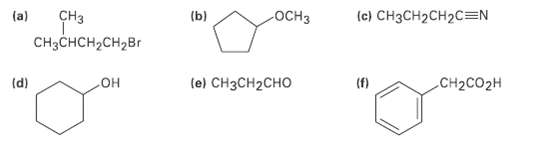
Transcribed Image Text:
(ы CHз (a) ОCHЗ (c) CH3CH2CH2C=N CH3CHCH,CH2Br le) CHзCH2CHO (d) (f) CH2CO2H он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
d CH3CHCHCHCHBr CHOH CHOCH3 ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Draw a structure for each of the following compounds in its more stable chair conformation. Explain your choice. (a) (b) CH3 CH3 CH (CH),C CHA CH, ," CH3 CH3
-
Draw enantiomers for each of the following compounds using: a. perspective formulas b. Fischer projections CH CH 1. CHjCHCH2H 2. CICH2CH2CHCH2CH3 3. CHjCHCHCHj
-
Draw a planar structure for each of the following compounds using dashed or solid wedges to show the stereochemistry of substituent groups (a) trans - 1,3 - dimethylcyclohexane (b) ( I S,2R)- I...
-
What does the following code fragment print? String \(s=\) "He11o World"; s. toUpperCase(); s. substring (6, 11); StdOut.println(s);
-
What monthly rent must she charge for each apartment to break even? Felice bought a duplex apartment at a cost of $150,000. Her mortgage payments on the property are $940 per month, $121 of which can...
-
1. Why is it important for ConocoPhillips to increase the value of its shares for its investors? 2. Research ConocoPhillips. How close has it come to raising the desired $10 billion? ConocoPhillips,...
-
For each of the following separate cases, recommend a form of business organization. With each recommendation, explain how business income would be taxed if the owners adopt the form of organization...
-
John Sleaze and Mary Scum run Earnings Management, Inc., a consulting business. They have the following items in their product line: 1. A database that lists types of depreciable assets and the...
-
3. Suppose you are planning to save $1000 per year for 10 years in a fund earns 10%. Assume that the first payment you would made at the end of Year 8 and the final payment at the end of Year 17....
-
Procter & Gamble has been the leading soap manufacturer in the United States since 1879, when it introduced Ivory soap. However, late in 1991, its major rival, Lever Bros. (Unilever), overtook it by...
-
Draw a compound that: (a) Has nine primary hydrogens (b) Has only primary hydrogens
-
Give IUPAC names for the followingcompounds: H CHCH2CCH H CH CHH2CH2CH (b) (e) CHH2H2C CH (a) H-H H CH3CH2CHCH2CH,CHCH3 (e) CH H CHCH2CH2CHCH2CH CH CH-H>H3 (d)
-
Natural potassium is 0.0117% radioactive 40K, which decays by positron emission with a half-life of 1.26 10 9 years. If an average banana has 6.00 10 -1 g potassium in it, what is the radioactivity...
-
Lifestyle is how one enacts the self-concept. The way they would enact it is through buying luxury items which is the most premium iPhone. The latent reasons why people want an iPhone 15 all have to...
-
Make a Tows Matrix that assess the strengths, weakness, opportunities, and threats for Dannon based on the case study For typical corporate strategies under purpose of communication. Strengths 1) 2)...
-
Now that you've watched the lectures, The Abilene Paradox movie, and the Challenger Disaster Video, I'd like you to think for a moment about when you may have observed the Abilene Paradox or...
-
Ensuring that the projectadheres to the selected quality standard . Often, ensuring that the project work is done 'correctly' is as important as ensuring that the end result fulfills the project's...
-
Think about some career planning and development issues; for example, mergers and reorganization uncertainty, lack of upward mobility, getting managers to understand your career potential, and...
-
What are the key issues to consider when planning to use semi-structured or in-depth interviews? LO9
-
Use the T account for Cash below to record the portion of each of the following transactions, if any that affect cash. How do these transactions affect the companys liquidity? Jan. 2 Provided...
-
You decide to cool a can of soda pop quickly in the freezer compartment of a refrigerator. When you take out the can, the soda pop is still liquid; but when you open the can, the soda pop immediately...
-
A compound with molecular formula C4H8O has a strong IR absorption at 1730 cm-1. Its mass spectrum includes key peaks at m/z 44 (the base peak) and m/z 29. Propose a structure for the compound and...
-
In the mass spectrum of 2, 6-dimethyl-4-heptanol there are prominent peaks at m/z 87, 111, and 126. Propose reasonable structures for these fragment ions.
-
In the mass spectrum of 4-methyl-2-pentanone a McLafferty rearrangement and two other major fragmentation pathways occur. Propose reasonable structures for these fragment ions and specify the m/z...
-
Calculate Social Security taxes, Medicare taxes and FIT for Jordon Barrett. He earns a monthly salary of $11,100. He is single and claims 1 deduction. Before this payroll, Barretts cumulative...
-
Bass Accounting Services expects its accountants to work a total of 26,000 direct labor hours per year. The company's estimated total indirect costs are $ 260,000. The company uses direct labor hours...
-
The Balance Sheet has accounts where the accountant must make estimates. Some situations in which estimates affect amounts reported in the balance sheet include: Allowance for doubtful accounts....

Study smarter with the SolutionInn App


