Show the hybridization at each of the atoms, except H, in these molecules. Indicate the type of
Question:
Show the hybridization at each of the atoms, except H, in these molecules. Indicate the type of each designated bond and the orbital's that are overlapping to form it?
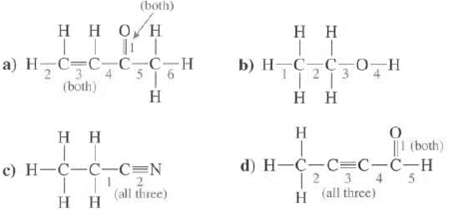
Transcribed Image Text:
(both) Η Η Ο Η TTT a) HC=C_C_CH Το (both) H Η Η 0 H-C-C-C=N 11 Η Η 2 (all three) Η Η || b) H_C_CO Η Η Η H |1 (both) d) H=C=C=C-C-H 1 2 3 4 H (all three)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (18 reviews)
a 1 3 5 Csp2Osp2 and TTC2p02p 2 OCsp2H1s Csp2Csp2 and TTC2pCp2 4 OCsp2Csp2 Csp2...View the full answer

Answered By

Loise Ndungu
I have five years of experience as a writer. As I embark on writing your papers from the prologue to the epilogue, my enthusiasm is driven by the importance of producing a quality product. I put premium product delivery as my top priority, as this is what my clients are seeking and what makes me different from other writers. My goal is to craft a masterpiece each time I embark on a freelance work task! I'm a freelance writer who provides his customers with outstanding and remarkable custom writings on various subjects. Let's work together for perfect grades.
4.90+
82+ Reviews
236+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
In the hydrocarbon (a) What is the hybridization at each carbon atom in the molecule? (b) How many Ï bonds are there in the molecule? (c) How many Ï bonds? (d) Identify all the 120o bond...
-
Indicate the type of atomic orbitals that are overlapping to form each of the different kinds of bonds in CH3OCH3 (For example, a carbon sp3 AO and a hydrogen Is AO). What kinds of orbitals are...
-
What is the hybridization at each C in this molecule? Indicate the type of bond and the orbital's that are overlapping to form it for each of the designated bonds? ITT H=C=C=C=C_C7H (both) H tall...
-
The general term that refers to the tendency of a parcel of air to either remain in place or change its initial position is ________. a. adiabatic b. conditional instability c. stasis d. stability
-
Explain why it may not be desirable for head office management to dictate transfer prices. Can you provide an example of a situation where this may be appropriate?
-
Lu Corp. erected and placed into service an offshore oil platform on January 1, 2017 at a cost of $10 million. Lu is legally required to dismantle and remove the platform at the end of its nine-year...
-
Bayfield Mud Company case. The text Quantitative Analysis of Management (Pearson, 2015) presents the case of the Bayfield Mud Company. Bayfield supplies boxcars of 50-pound bags of mud treating...
-
Nettles, King, and Tanaka are partners sharing income 3:2:1. After the firm's loss from liquidation is distributed, the capital account balances were: Nettles, $15,000 Dr.; King, $46,000 Cr.; and...
-
Payday loans are very short-term loans that charge very high interest rates. You can borrow $500 today and repay $535 in two weeks. What is the compound annual rate implied by this percent rate...
-
Allie has bought a new apple orchard. The orchard has a single file of trees, numbered from 1 to N. Each tree has a certail number of ripe apples. Allie has a rule she wants to follow. She wants to...
-
What is the hybridization at all atoms, except hydrogen's in these compounds? a) CHNH, d) b) CH=CHCHC=N OH 6 H NH
-
What is the hybridization at all atoms, except hydrogen's, in these compounds? a) H H H H H H H H CH 4.6 c) HC-N-CH3 : : g) CHC-OH h) CHC-NHCH,
-
A nurse is preparing to administer a prescribed cardiac glycoside to a patient based on the understanding that this group of drugs acts in which way? a. They block the sympathetic nervous system. b....
-
The COVID pandemic has created a crisis for many restaurateurs. The author of one of this week's readings has a suggestion that he thinks could help restaurants survive the crisis. Read the article...
-
Evidence is used to make a decision whenever the decision follows directly from the evidence (Tingling & Brydon, 2010). This is where so many people get it wrong or going by their personal beliefs or...
-
Pick 2 countries, find the price of a Big Mac in each country (if you want to pick another good/service, go ahead), express the price in the local currency, then with the help of exchange rate,...
-
Your task is to educate the public about the role of the Fed in the economy. Role: You are an economic issues reporter for PBS. Audience: Television audience of The Newshour on PBS Situation: Your...
-
Trade Queens Limited is a highly successful FMCG in Zambia. Salient points from the Year-end report indicate the following: Operating profit for the 2022 financial year is up 60% year on year,...
-
Discuss each of the needs in order within Maslows Need Hierarchy Theory.
-
Why do markets typically lead to an efficient outcome for buyers and sellers?
-
Prove that e x is equal to the sum of its Maclaurin series.
-
Draw structures for the following: (a) 2-Methyiheptane (b) 4-Ethyl-2, 2-dimethylhexane (c) 4-Ethyl-3, 4-dimethyloctane (d) 2, 4, 4-Trimethylheptane (e) 3, 3-Diethyl-2, 5-dimethylnonane (f)...
-
Draw a compound that: (a) Has only primary and tertiary carbons (b) Has no secondary or tertiary carbons (c) Has four secondary carbons
-
Draw a compound that: (a) Has nine primary hydrogens (b) Has only primary hydrogens
-
How to solve them..equation and explain ..please.. 1. Selected information from the companys financial records is presented below Equipment, December 31, 2013 $300,000 Equipment, December 31, 2014...
-
During 2024, its first year of operations, Hollis Industries recorded sales of $11,900,000 and experienced returns of $760,000. Cost of goods sold totaled $7,140,000 (60% of sales). The company...
-
What is the value of a 15% coupon bond with 11% return? Is it a discount or a premium bond?

Study smarter with the SolutionInn App


