What is the hybridization at all atoms, except hydrogen's in these compounds? a) CHNH, d) b) CH=CHCHC=N
Question:
What is the hybridization at all atoms, except hydrogen's in these compounds?
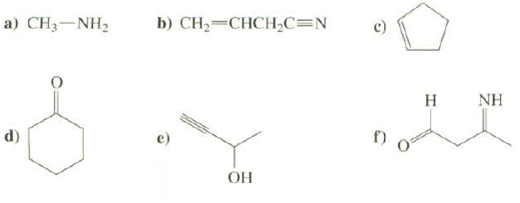
Transcribed Image Text:
a) CHạ—NH, d) b) CH₂=CHCH₂C=N OH 6 H NH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
a CH3NH2 sp sp d Sp3 sp3 O s...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
In the hydrocarbon (a) What is the hybridization at each carbon atom in the molecule? (b) How many Ï bonds are there in the molecule? (c) How many Ï bonds? (d) Identify all the 120o bond...
-
What is the hybridization at the indicated atoms in these compounds? a) CHCH=CH_NHCH3 12 3 c) CH,=CH0CH, 1 2 3 4 5 0: b) CHC0CH, 1 d) 2 1 NH
-
What is the hybridization of the indicated atom in each of the following molecules? a. b. c. d. e. f. CH CH CH CH CCH CH3CH2OH CH3CH NCH3 CH:OCH CH
-
y Working this on the www.bis.doc.gov website. "MY company makes fingerprinting powder kits for both domestic and external clients. It is a popular item in my inventory. I have a new customer in...
-
Explain why some organizations might prefer to use variable cost rather than absorption cost as a basis for setting transfer prices?
-
(a) During an ice skating performance, an initially motionless 80.0-kg clown throws a fake barbell away. The clown's ice skates allow her to recoil frictionlessly. If the clown recoils with a...
-
Quality control for irrigation data. Most farmers budget water by using an irrigation schedule. The success of the schedule hinges on collecting accurate data on evapotranspiration (ETo), a term that...
-
In Section 5.1 data envelopment analysis was used to evaluate the relative efficiencies of four hospitals. Data for three input measures and four output measures were provided in Tables 1 and 2....
-
Read the article, "John D.R. Leonard, Plaintiff, -against- PepsiCo, Inc., Defendant," found on the website provided in the Learn materials. Then answer the following questions: The court ruled that...
-
A molded plastic product (p = 1200 kg/m 3 c = 1500 J/kg K, k = 0.30 W/m K) is cooled by exposing one surface to an array of air jets, while the opposite surface is well insulated. The product may...
-
Show the location of the two planar nodes in this 3d atomic orbital?
-
Show the hybridization at each of the atoms, except H, in these molecules. Indicate the type of each designated bond and the orbital's that are overlapping to form it? (both) TTT a) HC=C_C_CH ...
-
Figure 25. 13 shows both the electric field lines and the equipotentials associated with the given charge distribution. (a) Is the potential at point A higher than, lower than, or the same as the...
-
I Need Hr project on Employee Engagement What is Employee Engagement and how does it contribute to organizational success? What is the role of HR in improving employee engagement? What are some...
-
In the United States, the Veterans Administration (VA) is tasked with, among other things, providing quality health care for U.S. military veterans. Chronically underfunded, the agency was having...
-
Using the keywords you brainstormed earlier in the module, conduct three separate searches in CQ Researcher - SAGE, Academic Search Ultimate, or another relevant database. When conducting these...
-
TechEx Repair allows local hardware stores to expand their service offerings to their customers by providing an off-site small engine repair service. Customers bring in small engines such as lawn...
-
Purpose: Sometimes we are asked to collaborate with a team of writers. This collaboration can help us to understand how others think differently from us and help us to think more creatively. This...
-
Identify three sources of motivation.
-
Trade credit from suppliers is a very costly source of funds when discounts are lost. Explain why many firms rely on this source of funds to finance their temporary working capital.
-
Is the series convergent or divergent? 22"31-n n=1
-
Draw and name all monochloro derivatives of 2, 5-dimethylhexane, C8H17C1.
-
Predict the hybridization of the carbon atom in each of the following functional groups: (a) Ketone (b) Nitrile (c) Carboxylic acid
-
Draw the structures of the following molecules: (a) Biacetyl, C4H6O2, a substance with the aroma of butter; it contains no rings or carboncarbon multiple bonds. (b) Ethylenimine, C2H5N, a substance...
-
Lakeland Inc. manufactured 2,500 units during the month of March. They incurred direct materials cost of $58,000 and overhead costs of $40,000. If their per-unit prime cost was $32.00 per unit, how...
-
Present Value Computations Using the present value tables, solve the following. ( Click here to access the PV and FV tables to use with this problem. ) Round your answers to two decimal places....
-
A company provided the following data: Sales $887,000 Variable costs $546,800 Fixed costs $310,000 Expected production and sales in units 36,000 What is the break-even point in sales dollars? Please...

Study smarter with the SolutionInn App


