Calculate E 0 for the process Cu(NH3)42+ + Cu(NH3)2 + 2 NH; given that Cut + 2NH3Cu(NH3)2
Question:
Calculate E0 for the process
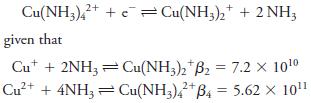
Transcribed Image Text:
Cu(NH3)42+ + Cu(NH3)2 + 2 NH; given that Cut + 2NH3Cu(NH3)2 B2 = 7.2 X 10¹⁰ 2+ Cu²+ + 4NH3 = Cu(NH3)42+ B4 = 5.62 × 10¹¹
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (16 reviews)
E0 for the process CuNH3 2 CuNH 3 2 2 NH3 can be calculated using the standard red...View the full answer

Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:
Students also viewed these Sciences questions
-
(a) Calculate E0 for the process (b) Use the shorthand notation to describe a cell consisting of a saturated calomel reference electrode and a silver indicator electrode that could be used to measure...
-
Compute E0 for the process ZnY2- + 2e- Zn(s) + Y4- Where Y4- is the completely deprotonated anion of EDTA, The formation constant for ZnY2- is 3.2 1016.
-
The solubility product for Pb 3 (AsO 4 ) 2 is 4.1 x 10 -36 . Calculate E 0 for the reaction Pb(AsO4)2(s) + Ge = 3Pb(s) + 2AsO4-
-
Express the quantity of 3.225 kJ in calories.
-
X = + zs; x = 58.98, = 8.7, s = 2.6 (value of an observation)
-
Arrange six departments into a 2 3 floor grid so that these conditions are satisfied: 1 is close to 2:5 is close to 2,5, and 6: and 3 is not close to 1 or 2.
-
5. Given nonzero numbers xo, Yo, un, va, So, to that satisfy the simultaneous equations (*) u2 + sx + ty = 0 v2 + tx + sy = 0 2s2x + 2t2y - 1 = 0 S2X - t2y = 0, prove that there exist functions u(x,...
-
Toys R Us sells a variety of childrens toys, games, books, and accessories. Assume that a local store has the following amounts for the month of March 2015. Required: 1. Prepare a multiple-step...
-
Current Attempt in Progress Marigold Company has the following account balances: The cost of goods purchased for the period is $86800. $103600. $93800. $80600
-
Tyler Company acquired all of Jasmine Companys outstanding stock on January 1, 2019, for $206,000 in cash. Jasmine had a book value of only $140,000 on that date. However, equipment (having an...
-
Briefly explain why the sparingly soluble product must be removed by filtration before you back-titrate the excess silver ion in the Volhard determination of (a) Chloride ion. (b) Cyanide ion. (c)...
-
Write chemical formulas for the following complex ions: (a) Hexamminezinc(II) (b) Dichloroargentate (c) Disulfatocuprate(II) (d) Trioxalatoferrate(III) (e) Hexacyanoferrate(II)
-
Two hundred volunteers are recruited for a study of how Internet shopping affects purchases. Each person is allowed to choose whether to be in the Internet user group or the group that agrees not to...
-
Question TARIMAX MASTO Copper Explorations recently acquired the rights to mine a new site. Machinery, equipment and a truck were purchased to begin the mining operations at the site. Details of the...
-
Exercise 6 - 6 ( Algo ) The Town of Weston has a Water Utility Fund with the following trial balance as of July 1 , 2 0 2 3 , the first day of the fiscal year: During the year ended June 3 0 , 2 0 2...
-
The University of Cincinnati Center for Business Analytics is an outreach center that collaborates with industry partners on applied research and continuing education in business analytics. One of...
-
What is the correct answer to this? SQL QUESTION Sales Data for All Customers and Products Write a query that will return sales details of all customers and products. The query should return all...
-
Below are the jersey numbers of 11 players randomly selected from a football team. Find the range, variance, and standard deviation for the given sample data. What do the results tell us? 84 18 34 3...
-
What is the AIDA Model? How does the AIDA model facilitate the planning and execution of marketing communications?
-
7 A 29-year-old, previously healthy man suddenly collapses at a party where legal and illicit drugs are being used. Enroute to the hospital, he requires resuscitation with defibrillation to establish...
-
Suggest an efficient synthesis for each of the following transformations: a. b. c. d. Br Br
-
Identify the reagents necessary to achieve each of the following transformations: Br Br Br Br Br
-
Determine the configuration for every chirality center in each of the following compounds. a. b. c. HO H- - OH H,OH
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...

Study smarter with the SolutionInn App


