List the positions of the important absorption bands in the IR spectra of these compounds: b) H,H-CH-C3DN
Question:
List the positions of the important absorption bands in the IR spectra of these compounds:
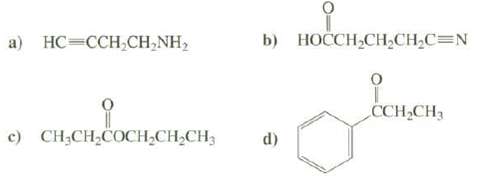
Transcribed Image Text:
b) НОССH,СH-CH-C3DN а) НС-ССH,СH,NH> CCH,CH3 c) CH;CH,COCH;CH,CH3 d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
a NH 2 two bands 34003250 cm 1 CH 3300 cm 1 CH 30002850 cm1 CC ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The IR and mass spectra for three different compounds are shown in Figures 13.45-13.47. Identify each compound. a. b. c. 25 26 2.7 28 19 2000 100 43 58 100 71 85 0 20 40 60 80 100 120 m/z 13 14 15 6...
-
Predict the important absorptions in the IR spectra of these compounds.
-
The IR spectra shown next may include a carboxylic acid, an ester, an amide, a nitrile, an acid chloride, or an acid anhydride. Determine the functional group suggested by each spectrum, and list the...
-
Fill in the missing mass number and atomic number for each of these particles or types of radiation. alpha (?) He beta (?) e gamma y neutron n
-
At the time of its liquidation under 332, Pink Corporation (earnings and profits of $560,000) had the following assets and liabilities: cash ($175,000); marketable securities (fair market value of...
-
A company borrows $2.6 million for 15 years at 5.6%, compounded quarterly. After 2 years of regular payments, the company's profits are such that management feels it can increase the quarterly...
-
Recall that the variance of a binomial sample proportion, F, depends on the value of the population parameter,~. As a consequence, the variance of a sample percentage, (loop)%, also depends on p....
-
The deepest point in the ocean is in the Mariana Trench, about 11 km deep. The pressure at this depth is huge, about 1.13 - 108 N/m2. (a) Calculate the change in volume of 1.00 m3 of seawater carried...
-
Milano Pizza is a small neighborhood pizzeria that has a small area for in-store dining as well as offering take-out and free home delivery services. The pizzerias owner has determined that the shop...
-
There is a lottery with n coupons and n people take part in it. Each person picks exactly one coupon. Coupons are numbered consecutively from 1 to n, n being the maximum ticket number. The winner of...
-
Explain how IRD spectroscopy could be used to distinguish between these compounds: CH;CH,C=CH a) CH,CH,CH=CH; and CH3 H- b) CH, nd CH3 CCH3 CH c) and d) CH.CH,CH,C,NH, and CH.CH,NHCH,CH,
-
Explain how IR spectroscopy could be used to distinguish between thesecompounds: and b) and NH2 d d) and and e) ) and
-
Consider the following questions about glutamate dehydrogenase. (a) The reaction as shown on page 569 has NH3 as a reactant, instead of NH4+, which is far more abundant at physiological pH. Why is...
-
The problem I have identified is that healthcare leaders could benefit from addressing the issue of stress and burnout, which impact revenue (Scott, 2022). I have found a peer-reviewed article...
-
Facebook, Inc is the company Complete a 3-5 year forecast for your target company assuming a 10% average growth rate for the duration of the forecast period Assuming a long-term growth rate of 5%...
-
BSC-It is important for healthcare leaders to link their departmental balanced scorecard (BSC) to a corporate BSC because it facilitates alignment with the overall strategic objectives of the...
-
Hebert Company adds material at the beginning of production. The following production information is available for March: Beginning Work in Process Inventory (40% complete as to conversion) Started...
-
What modifications would you suggest the leaders of the steel organization when dealing with the use of more efficient technology, carbon emissions, and negative economic impacts in order tomake in...
-
critically discuss control in managing operations.
-
DEPARTMENT DATA EMPLOYEE DATA EmployeeNumber FirstName Mary Rosalie Richard George Alan 3 4 5 7 8 9 855555ES 12 13 14 15 16 17 Create the database tables in SQL or ACCESS: 18 19 20 PROJECT DATA Ken...
-
Not only can the internet be used as an information-gathering tool, but it can also be an effective way for firms to advertise their products. In 2001, advertising of all types decreased, but online...
-
The polymeric resin used for Merrifield solid-phase peptide synthesis (Section 26.8) is prepared by treating polystyrene with N-(hydroxymethyl) phthalimide and trifluoromethanesulfonic acid, followed...
-
2-Ethyl-1-hexanol, used in the synthesis of di (2-cthythcxyl) phthalate plasticizer, is made commercially from butanal. Show the likely synthesis route.
-
One of the steps in fat metabolism is the reaction of glycerol (1, 2, 3-propanetriol) with ATP to yield glycerol 1-phosphate. Write the reaction, and draw the structure of glycerol 1-phosphate.
-
THIS IS ONE QUESTION WITH TWO PARTS. PLEASE ANSWER COMPLETELY AND SHOW ALL WORK. (NO EXCEL) Information for Question 1: State Probability Retum on A Return on B Return on C Retum on Portfolio X Boom...
-
Direct materials (5.0 Ibs. @ $5.00 per Ib.) Direct labor (2.0 hrs. @ $13.00 per hr.) Overhead (2.0 hrs. @ $18.50 per hr.) Total standard cost $25.00 26.00 37.00 $88.00 The predetermined overhead rate...
-
Problem 1-28 (Algo) (LO 1-4, 1-5, 1-6b 1-7) Harper, Inc., acquires 40 percent of the outstanding voting stock of Kinman Company on January 1, 2020, for $316,100 in cash. The book value of Kinman's...

Study smarter with the SolutionInn App


