Indicate the positions of the absorption bands and any other noteworthy features in the hydrogen region of
Question:
Indicate the positions of the absorption bands and any other noteworthy features in the hydrogen region of the IR spectra of thesecompounds:
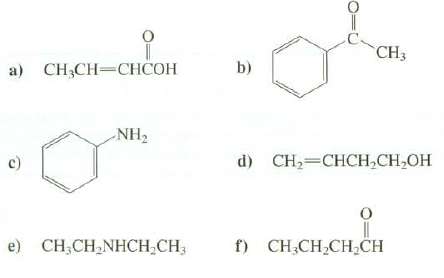
Transcribed Image Text:
CH3 а) CН,CH—СНСОН b) NH2 d) CH2=CHCH,CH,OH f) CH,CH,CH,CH e) CH;CH,NHCH;CH;
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
a OH 3000 cm 1 very broad CH 31003000 cm 1 CH 30002850 cm 1 ...View the full answer

Answered By

Ayush Jain
Subjects in which i am expert:
Computer Science :All subjects (Eg. Networking,Database ,Operating System,Information Security,)
Programming : C. C++, Python, Java, Machine Learning,Php
Android App Development, Xamarin, VS app development
Essay Writing
Research Paper
History, Management Subjects
Mathematics :Till Graduate Level
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the positions of the absorption bands in the IR spectra for the carbonyl groups of thesecompounds. HO. b) CH,CH-CHCH (p
-
For the following reaction profiles, indicate The positions of reactants and products. The activation energy. ÎE for the reaction. The second reaction profile is representative of a reaction...
-
The IR and mass spectra for three different compounds are shown in Figures 13.45-13.47. Identify each compound. a. b. c. 25 26 2.7 28 19 2000 100 43 58 100 71 85 0 20 40 60 80 100 120 m/z 13 14 15 6...
-
Determine the number of valence electrons for each of thefollowing four elements. Part A Ga _______ Express your answer as an integer. Part B Pb ________ Express your answer as an integer. Part C Cl...
-
Chase Opportunity has selected you as the project manager to oversee their latest project - Chase Retail Complex. Chase's vision is to build four (4) 10,000 square foot buildings (see concept drawing...
-
If $4000 is deposited at the end of each half year in an account that earns 6.2% compounded semiannually, how long will it be before the account contains $120,000?
-
In Exercise 7.29 (p. 371), the inflation forecasts of nine economists that were made in June 1999 and January 2000 were reported. These forecasts, obtained from the Wall Street Journal, are...
-
A continuous distillation operation with a reflux ratio (L/D) of 3.5 yields a distillate containing 97 wt% B (benzene) and bottoms containing 98 wt% T (toluene). Due to weld failures, the 10 plates...
-
FND Hill has two options to enter the small business market through a partnership or through direct sales. The costs and pricing differs for each approach. Hill Partnership: - Sales price is 3 5 %...
-
You've been given the critical assignment of selecting the site for your company's new plant. After months of negotiations with landowners, numerous cost calculations, and investments in ecological,...
-
Explain which of these bonds has the absorption for its stretching vibration at higher wave number: (a) C H or C D (b) C = C or C C (c) C C1 or C I
-
Explain why the presence of a triple bond is much easier to detect in the IR spectrum of 1-hexyne than it is in the spectrum of3-hexyne. 80 60 O The sp-hybridized CH absorption bands: from 3000-2850...
-
Using more debt lowers profits and thus the ROA. Why doesnt debt have the same negative effect on the ROE? AppendixLO1
-
(Reference: A Closer Look on Cost Accounting, De Jesus, 2019) Paulo Corporation had the following account balances as of August 1, 2020: Raw materials inventory (direct and indirect) Work in Process...
-
Use the given data values (a sample of female arm circumferences in centimeters) to identify the corresponding z scores that are used for a normal quantile plot, then identify the coordinates of each...
-
1. What stakeholders other than customers, suppliers, and partners should be considered when conceiving ways to monetize data? 2. What would be one strategy to maximize "reliability" attribute in...
-
What important guidance does little's law offer healthcare leadership regarding the planning and utilization of resources
-
Semester Two Practice Examinations, 2022 STAT2201 Question 2. [10 marks] A study investigated the effect of playing computer games on heart rate. Twenty eight individuals were recruited into the...
-
How will international cricket look in 20 years following the success of the IPL?
-
A bar of length = 1 has one fixed and one free end and stiffness function c(x) = 1 - x. Find the displacement when subjected to a unit force. Pay careful attention to the boundary condition at the...
-
In 1990, the four-firm concentration ratio in the U.S. textbook industry was about 40 percent. The top four firms had an average of about 10 percent of the textbook market. The other 60 percent of...
-
Propose a mechanism to account for the formation of Bakelite from acid-catalyzed polymerization of phenol and formaldehyde.
-
Identify? the structural class to which the following polymer belongs, and show the structure of the monomer units used to make it:
-
Show the structures of the polymers that could be made from the following monomers (yellow-green =Cl): (b) (a)
-
*Prepare the plant assets section of Amphonie's balance sheet at December 31, 2021 using the information below. At December 31, 2020, Amphonie Company reported the following as plant assets. Land $...
-
Question 1 of 1 - / 100 View Policies Current Attempt in Progress Pargo Company is preparing its budgeted income statement for 2020. Relevant data pertaining to its sales, production, and direct...
-
Schopp Corporation makes a mechanical stuffed alligator that sings the Martian national anthem. The following information is available for Schopp Corporation's anticipated annual volume of 500,000...

Study smarter with the SolutionInn App


