Explain why the presence of a triple bond is much easier to detect in the IR spectrum
Question:
Explain why the presence of a triple bond is much easier to detect in the IR spectrum of 1-hexyne than it is in the spectrum of3-hexyne.
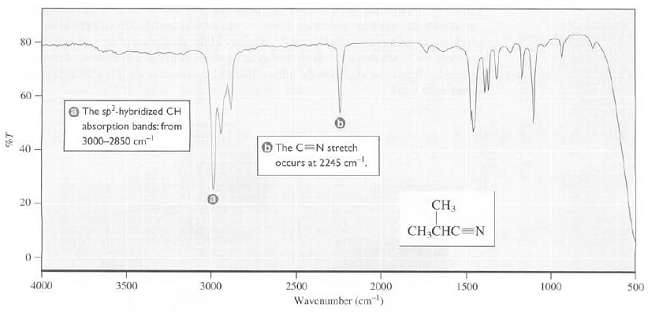
Transcribed Image Text:
80 60 O The sp-hybridized CH absorption bands: from 3000-2850 cm O The CN stretch occurs at 2245 cm. 40 20 CH CH,CHC=N 4000 3500 3000 2500 2000 1500 1000 500 Wavenumber (cm)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
The band in the triple bond region at 21502100 ...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
A test for the presence of a certain disease has probability .20 of giving a false-positive reading (indicating that an individual has the disease when this is not the case) and probability .10 of...
-
Explain why the presence of a charismatic leader tends to enhance the job satisfaction of group members.
-
The shaft of a generator is much easier to turn when the generator is not connected to an outside circuit than when such a connection is made. Why?
-
Which power plant has high load factor?
-
The Stoney Company sells many products. Wolie is one of its popular items. Below is an analysis of the inventory purchases and sales of Wolie for the month of March. Stoney Company uses the periodic...
-
If $2000 is deposited at the end of each quarter into an account that earns 6% compounded quarterly, how long until the account reaches $50,000? (a) State whether the problem relates to an ordinary...
-
According to the National Sleep Foundation, companies are encouraging their workers to take "power naps" (Athens Daily News, Jan. 9,2000). In Exercise 7.27 (p. 371), you analyzed data collected by a...
-
Restate persuasively each of the following issues. Each issue should be redrafted twicepersuasively from the view of the opposing sides. A. Under the provisions of the exclusionary rule, should...
-
Its questions 1,2,& 3 1. Compute total variable cost per unit. 2. Compute total fixed costs. 3. Prepare a flexible budget at activity levels of 12,000 units and 16,000 units
-
Write and test a program to simulate the flow diagram of CSMA/CD in Figure 12.13. Figure 12.13 Flow diagram for the CSMA/CD Station has a frame to send K= 0 Legend T Frame average transmission time...
-
Indicate the positions of the absorption bands and any other noteworthy features in the hydrogen region of the IR spectra of thesecompounds: CH3 ) C,CH b) NH2 d) CH2=CHCH,CH,OH f) CH,CH,CH,CH e)...
-
The exhaust from a poorly maintained automobile may contain a wide variety of different hydrocarbon pollutants. Why is the 3000 to 2900 cm1 region a good place to monitor the amount of these...
-
Design a questionnaire for determining businesses' selection criteria for choosing PC and network communications products.
-
Time ( s ) Velocity ( m / s ) 1 2 3 4 5 6 7 8 Calculate the velocity
-
The table below gives the data about Etruria's balance of payments. (All figures are in billions of dollars.) Foreign investment in Etruria Secondary (transfers) income received from abroad Primary...
-
Olive Corporation buys a material for P20 per unit. Sixteen thousand parts a year are needed. Carrying costs is P3.00 per unit and the ordering cost is P15. Required: Compute the economic order...
-
As a healthcare leader or manager, most of us are charged with supervising employees. The literature suggests the importance of hiring and retaining employees with high levels of emotional...
-
7-8. Evaluate the sum exactly. (10 points each) 7. 18 (1) n (33) "
-
What are the financial implications for the future of individual sports sponsorship arising from the Tiger Woods scandal?
-
Show that if A is any m n matrix, then Im A = A and AIn = A.
-
In 2008, the German-owned DHL Express decided that it could no longer compete with FedEx and UPS in the U.S. domestic package delivery market. The company had lost more than $1 billion a year in 2006...
-
Identify the monomer units from which each of the following polymers is made, and tell whether each is a chain-growth or a step-growthpolymer. (b) +CF2-CFCI (c) NHCH2CH2CH2C (a) +CH2-0+o (d) (e)
-
Draw a three-dimensional representation of segments of the following polymers: (a) Syndiotactic poly acrylonitrile (b) Atactic poly (methyl methacrylate) (c) Isotactic poly (vinyl chloride)
-
Draw the structure of Kodel, a polyester prepared by heating dimethyl 1, 4-henzcnedicarhoxylate with 1, 4-bis (hydroxymethyl)cyclohexane. H? -CH2 1,4-Bis(hydroxymethyl)cyclohexane
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


