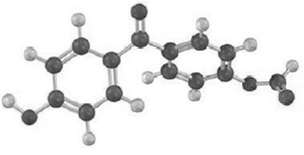
Identify? the structural class to which the following polymer belongs, and show the structure of the monomer
Question:
Identify? the structural class to which the following polymer belongs, and show the structure of the monomer units used to make it:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
cc oo co The poly...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which monomer and which type initiator would you use to synthesize each of the following polymers? a. b. c. ¬CH2CH2OCH2CH2O¬ d. CHs CH3 CHs CH3 CH3 CH3 CH,CH-CH-CH- CH CHCH CH COCH COCH
-
Identify the major structural features of class I and class II MHC proteins.
-
The Mann Company belongs to a risk class for which the appropriate discount rate is 10 percent. Mann currently has 220,000 outstanding shares selling at $110 each. The firm is contemplating the...
-
apple company: 1.what are key characteristics of the industry? 2. where is the company in its life cycle?
-
How can each one of the business-level strategies be used to position the firm relative to the five forces of competition in a way that helps the firm earn above-average returns? Discuss.
-
Suppose you are given the following information on the S&P 500: Date Close 10/1/2015 .....................1,923.82 10/2/2015 .....................1,951.36 10/5/2015 .....................1,987.05...
-
What are the stages in the buying process?
-
Use the information from BE17-1, but assume the bonds are purchased as an available-for-sale security. Prepare Garfields journal entries for (a) The purchase of the investment, (b) The receipt of...
-
3 On January 1, 2020, Indiana Jones created a new moving company called Raiders Moving Co. His sons worked for him as drivers for the company. These activities occurred during the company's first...
-
The COVID-19 pandemic affected companies in the food industry in unique ways, particularly during 2020. Ruth's Hospitality Group (Ticker: RUTH) develops and operates fine dining restaurants under the...
-
Propose a mechanism to account for the formation of Bakelite from acid-catalyzed polymerization of phenol and formaldehyde.
-
Show the structures of the polymers that could be made from the following monomers (yellow-green =Cl): (b) (a)
-
In your opinion, what communication method would be ideal for an organization that has offices in many different countries?
-
how is lateral force(fy) determined from this data Tyre Responses 1 1 1 1 1 1.3 1.3 1.3 1.3 1.3 1.6 1.55 1.45 1.27 1.1 Fz (N) 0 400 800 1200 1500 Slip Angle (deg) Fy1 (N) Fy2 (N) Fy3 (N) 0.0 0 0 0.5...
-
(13%) Problem 8: A wire is oscillated to create a wave of the form y(x,t) = Asin(x - 30t) == The wave is reflected from a fixed end producing a reflection of the form y2(x,t) = A sin(x + 30t) The two...
-
Using the definitions of even integer and odd integer, give a proof by contraposition that this statement is true for all integers n: If 5n+3 is even, then n is odd.
-
7. Design the formwork for a wall 8-ft (2.44-m) high to be poured at the rate of 5 ft/h (1.53 m/h) at a temperature of 77F (25C). The concrete mixture will use Type I cement without retarders and is...
-
tempt in Progress The City of Minden entered into the following transactions during the year 2026. 1. A bond issue was authorized by vote to provide funds for the construction of a new municipal...
-
If a restaurant s turnover rate is 100 percent, what does that mean?
-
Write an essay describing the differing approaches of nursing leaders and managers to issues in practice. To complete this assignment, do the following: 1. Select an issue from the following list:...
-
The extent of reaction generally depends on pressure as well as temperature. For the reaction (or phase transition) the standard-state Gibbs energy change at 25C is 2866 J/mol. The density of...
-
Write a balanced chemical equation for the combustion of cyclohexane.
-
Using the data in Table 2.5, estimate the heat of combustion of Icosane (in kJ/mol) TABLE 2.5 Heats of Combustion (-AH) of Representative Alkanes Compound Formula kJ/mol kcal/mol Unbranched alkanes...
-
Without consulting Table 2.5, arrange the following compounds in order of decreasing heat of combustion: pentane, isopentane, neopentane, hexane. TABLE 2.5 Heats of Combustion (-AH) of Representative...
-
Consider a 5 year debt with a 15% coupon rate paid semi-annually, redeemable at Php1,000 par. The bond is selling at 90%. The flotation cost is Php50 per bind. The firm's tax bracket is 30%.
-
A project will generate annual cash flows of $237,600 for each of the next three years, and a cash flow of $274,800 during the fourth year. The initial cost of the project is $749,600. What is the...
-
You want to invest annual amounts over the next 15 years. If your goal is to have $15,000 at the end of that time and if you can earn 8 percent on your invested funds, how much do you need to invest...

Study smarter with the SolutionInn App


