Question: Pyrocatechol violet (Table 11-3) is to be used as a metal ion indicator in an EDTA titration. The procedure is as follows: 1. Add a
Pyrocatechol violet (Table 11-3) is to be used as a metal ion indicator in an EDTA titration. The procedure is as follows:
1. Add a known excess of EDTA to the unknown metal ion.
2. Adjust the pH with a suitable buffer.
3. Back-titrate the excess chelate with standard Al3+.
11-3
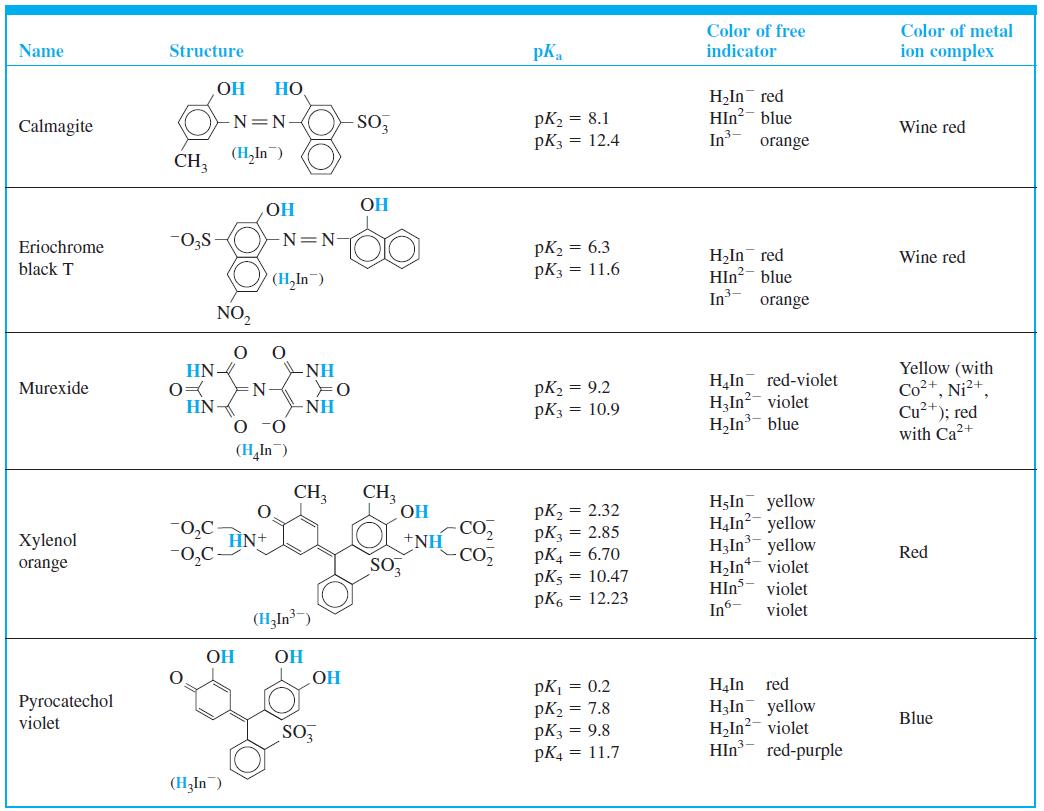
Color of free indicator Color of metal Name Structure pK, ion complex OH H,In red HIn?- blue In Calmagite -N=N SO, pK2 = 8.1 Wine red pK3 = 12.4 orange CH, (H,In) HO -N=N- pK2 = 6.3 pK3 = 11.6 Eriochrome H,In red HIn?- blue In Wine red black T (H,In) orange NO, Yellow (with Co2+, Ni?+, Cu+); red with Ca2+ HN- =N- -NH red-violet pK2 = 9.2 pK3 = 10.9 H,In H,In?- violet H,In blue Murexide -NH O -0 HN (H,In) CH3 CH, OH pK, = 2.32 pK3 = 2.85 pK = 6.70 pK5 = 10.47 pK6 = 12.23 HIn yellow H,In? yellow H,In yellow H,In* violet violet "0,C HN+ -CO, +NH CO Xylenol Red 0,C orange SO, HIns In- violet (H,In) OH OH pK = 0.2 pK2 = 7.8 pK3 = 9.8 pK4 = 11.7 H,In red H3In yellow H,In?- violet HIn H Pyrocatechol violet Blue SO red-purple (H,In)
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it
Pyrocatechol violet also known as Eriochrome Violet R is a type of metal ion indicator used in compl... View full answer

Get step-by-step solutions from verified subject matter experts


