Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Experiment #7 LIMITING REACTANT Name REPORT SHEET DATA Table A. Limiting Reactant Data Description Color of bromocresol purple in NaOH solution Measurement or Observation
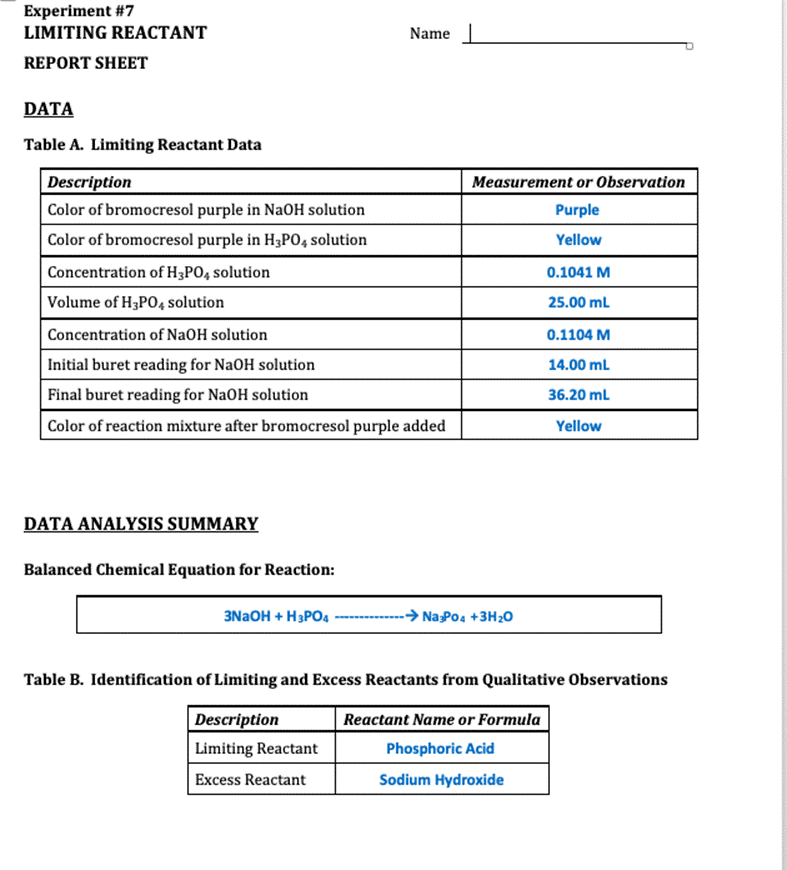
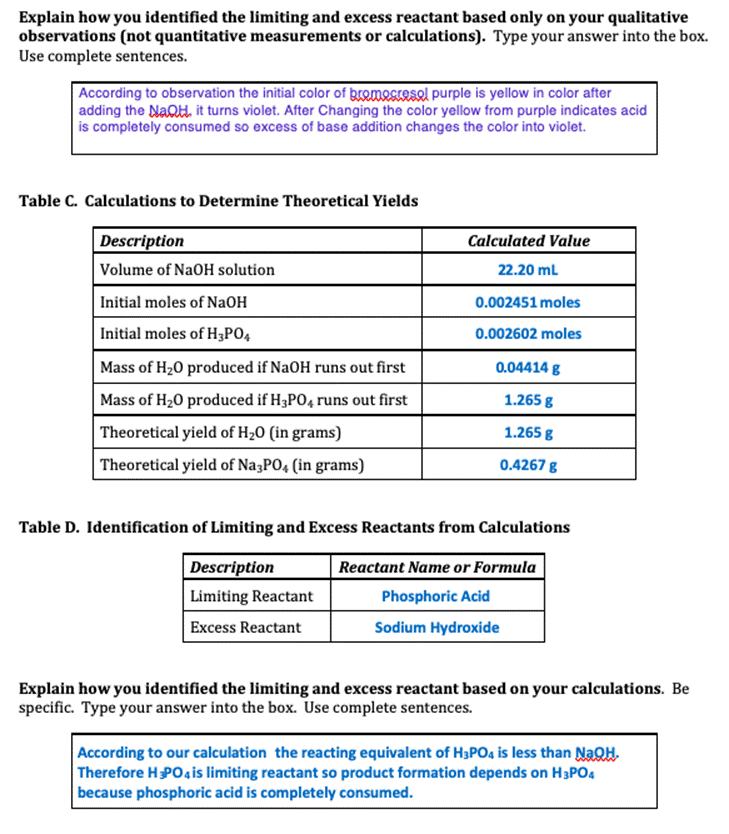
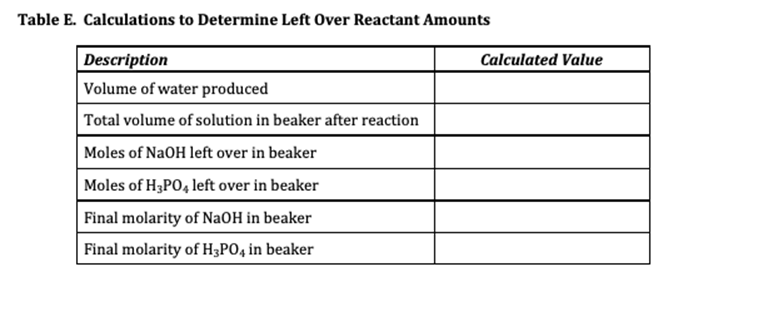
Experiment #7 LIMITING REACTANT Name REPORT SHEET DATA Table A. Limiting Reactant Data Description Color of bromocresol purple in NaOH solution Measurement or Observation Purple Color of bromocresol purple in H3PO4 solution Yellow Concentration of H3PO4 solution 0.1041 M Volume of H3P04 solution 25.00 mL Concentration of NaOH solution 0.1104 M Initial buret reading for NaOH solution 14.00 ml Final buret reading for NaOH solution 36.20 mL Color of reaction mixture after bromocresol purple added Yellow DATA ANALYSIS SUMMARY Balanced Chemical Equation for Reaction: 3NAOH + H3PO4 ------- - Na Po4 +3H20 Table B. Identification of Limiting and Excess Reactants from Qualitative Observations Description Reactant Name or Formula Limiting Reactant Phosphoric Acid Excess Reactant Sodium Hydroxide Explain how you identified the limiting and excess reactant based only on your qualitative observations (not quantitative measurements or calculations). Type your answer into the box. Use complete sentences. According to observation the initial color of gromocresel purple is yellow in color after adding the NaQH. it turns violet. After Changing the color yellow from purple indicates acid is completely consumed so excess of base addition changes the color into violet. Table C. Calculations to Determine Theoretical Yields Description Calculated Value Volume of NaOH solution 22.20 ml Initial moles of NaOH 0.002451 moles Initial moles of H3PO4 0.002602 moles Mass of H20 produced if NaOH runs out first 0.04414 g Mass of H20 produced if H3PO4 runs out first 1.265 g Theoretical yield of H20 (in grams) 1.265 g Theoretical yield of Na3PO4 (in grams) 0.4267 g Table D. Identification of Limiting and Excess Reactants from Calculations Description Reactant Name or Formula Limiting Reactant Phosphoric Acid Excess Reactant Sodium Hydroxide Explain how you identified the limiting and excess reactant based on your calculations. Be specific. Type your answer into the box. Use complete sentences. According to our calculation the reacting equivalent of H3PO4 is less than NaOH. Therefore HPO4 is limiting reactant so product formation depends on H3PO4 because phosphoric acid is completely consumed. Table E. Calculations to Determine Left Over Reactant Amounts Description Calculated Value Volume of water produced Total volume of solution in beaker after reaction Moles of NaOH left over in beaker Moles of H3PO4 left over in beaker Final molarity of NaOH in beaker Final molarity of H3PO4 in beaker
Step by Step Solution
★★★★★
3.33 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
9 Pou voleme Conc 01oUI M 25 mL 17 So mote 01041 A 24 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


