Question: How should we operate a mixed flow reactor so as to maximize the production of R? Separation and recycle of unused reactant is not practical.
How should we operate a mixed flow reactor so as to maximize the production of R? Separation and recycle of unused reactant is not practical.
When aqueous A and aqueous B (CA0 = CB0) are brought together they react in two possible ways: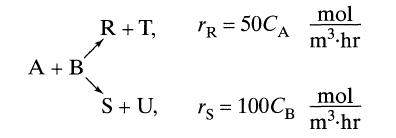
to give a mixture whose concentration of active components (A, B, R, S, T, U) is Ctotal = CA0 + CB, = 60 mol/m3. Find the size of reactor needed and the R/S ratio produced for 90% conversion of an equimolar feed of F = F = 300 mol/hr:
In a reactor that gives highest CR. This should be plug flow for A and side entry for B. In such a reactor introduce B in such a way that CB is constant throughout the reactor.
A + B R+T, S+ U, mol TR= 50A m.hr rs = 100CB mol m.hr
Step by Step Solution
3.39 Rating (158 Votes )
There are 3 Steps involved in it
The presented question details a scenario where two aqueous reactants A and B are combined and can react to form two different sets of products To address your question Ill break it down into several ... View full answer

Get step-by-step solutions from verified subject matter experts


