Question: Shows the temperatureconversion trajectory for a train of reactors with interstage heating. Now consider replacing the interstage heating with injection of the feed stream in
Shows the temperature–conversion trajectory for a train of reactors with interstage heating. Now consider replacing the interstage heating with injection of the feed stream in three equal portions, as shown in Figure P11-10A: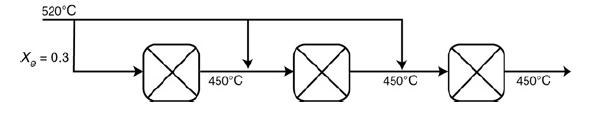
The figure shows an input feed of 520 degrees celsius supplied to three reactors. The three reactors are connected in series. A feed stream of 520 degrees celsius is supplied as the individual input of three reactors. The temperature of the output stream from each of these reactors is 450 degrees celsius. The adiabatic equilibrium conversion is 0.3. Sketch the temperature–conversion trajectories for
(a) An endothermic reaction with entering temperatures as shown,
(b) An exothermic reaction with the temperatures to and from the first reactor reversed, that is, T0 = 450°C.
520C X = 0.3 450C 450C 450C
Step by Step Solution
3.24 Rating (145 Votes )
There are 3 Steps involved in it
a For first reactor Feed Temperature to the reactor 2 is 5204502 485 K Feed Temperature to reactor 3 ... View full answer

Get step-by-step solutions from verified subject matter experts


