Question: The reaction A + B C + D is carried out adiabatically in a series of staged packed-bed reactors with interstage cooling. The lowest
The reaction A + B ⇄ C + D is carried out adiabatically in a series of staged packed-bed reactors with interstage cooling. The lowest temperature to which the reactant stream may be cooled is 27°C. The feed is equal molar in A and B, and the catalyst weight in each reactor is sufficient to achieve 99.9% of the equilibrium conversion. The feed enters at 27°C and the reaction is carried out adiabatically. If four reactors and three coolers are available, what conversion may be achieved?
Additional information: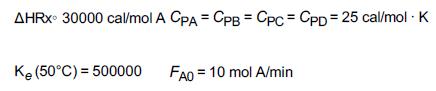
First prepare a plot of equilibrium conversion as a function of temperature.
AHRX 30000 cal/mol A CPA = CPB = CPC = CPD = 25 cal/mol. K Ke (50C) = 500000 FAO 10 mol A/min
Step by Step Solution
3.26 Rating (149 Votes )
There are 3 Steps involved in it
Kc CCCD CC 1x x KC 1K AHRX 30000 TTo 300 X 300600X C C 2525 See t... View full answer

Get step-by-step solutions from verified subject matter experts


