The aqueous decomposition of A is studied in an experimental mixed flow reactor. The results in Table
Question:
The aqueous decomposition of A is studied in an experimental mixed flow reactor. The results in Table P5.25 are obtained in steady-state runs. To obtain 75% conversion of reactant in a feed, CA0 = 0.8 mol/liter, what holding time is needed in a plug flow reactor?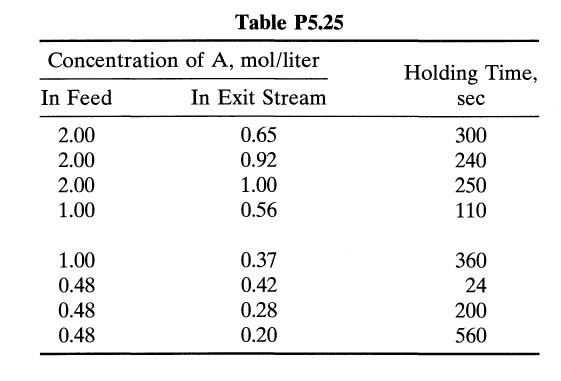
Transcribed Image Text:
Concentration In Feed 2.00 2.00 2.00 1.00 1.00 0.48 0.48 0.48 Table P5.25 of A, mol/liter In Exit Stream 0.65 0.92 1.00 0.56 0.37 0.42 0.28 0.20 Holding Time, sec 300 240 250 110 360 24 200 560
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To determine the required holding time in a plug flow reactor to achieve 75 conversion of reactant A ...View the full answer

Answered By

Prachi Goyal
No experience of tutoring and teaching.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Repeat the previous problem but for a mixed flow reactor. Previous Problem The aqueous decomposition of A is studied in an experimental mixed flow reactor. The results in Table P5.25 are obtained in...
-
The aqueous decomposition of A produces R as follows: The following results are obtained in a series of steady state runs, all having no R in the feed stream. From this kinetic information, find the...
-
The kinetics of the aqueous-phase decomposition of A is investigated in two mixed flow reactors in series, the second having twice the volume of the first reactor. At steady state with a feed...
-
QUESTION 7 We discussed the selection sort sorting algorithm. What is the worst case time complexity of selection sort when sorting a list of n elements? o(lg n) O(n) O(n lg n) O(n) O(log n) O(1)
-
The manager for Tyler Bank and Trust has the following assets and liabilities to manage: If the manager wants a duration gap of 3.00, what level of saving accounts should the bank raise? Assume that...
-
Required information Exercise 6-16 (Algo) Break-Even Analysis and CVP Graphing (LO6-2, LO6-4, LO6-5] [The following information applies to the questions displayed below.] The Hartford Symphony Guild...
-
Find the probability that a person aged 50 is still alive at age 70.
-
The daily demand for tuna sandwiches at an Ohio University cafeteria vending machine is 8, 9, 10, or 11, with probabilities 0.4, 0.3, 0.2, or 0.1, respectively. Assume the following random numbers...
-
QUESTION 1: Pretika Holdings Berhad is a property developer company in Malaysia, due to high demand of property, the company has decided to build up a new township which located at Bangi. The...
-
The data in Table P5.28 have been obtained on the decomposition of gaseous reactant A in a constant volume batch reactor at 100C. The stoichiometry of the reaction is 2A R + S. What size plug flow...
-
A high molecular weight hydrocarbon gas A is fed continuously to a heated high temperature mixed flow reactor where it thermally cracks (homogeneous gas reaction) into lower molecular weight...
-
For an interesting perspective on the historical development of cost and management accounting, refer to Johnson (1987), Johnson and Kaplan (1987). For a more recent reference see also Kannaiah...
-
Determine the amount that would be reported in ending merchandise inventory on October 15 using the FIFO inventory costing method. Enter the transactions in chronological order, calculating new...
-
QwikShare is a new not-for-profit organization that will rent low-emissions automobiles at QwikStops in suburban areas in order to provide an environmentally friendly transportation option to its...
-
Study customer touchpoints. Remember that we are working with the B2C market. Select two stages of the customer decision journey Answer the following questions: For each selected stage, identify a...
-
The following data from the just completed year are taken from the accounting records of Mason Company: Sales Direct labor cost Raw material purchases Selling expenses Administrative expenses...
-
Skysong, Inc. has issued three different bonds during 2022. Interest is payable annually on each of these bonds. 1. On January 1, 2022, 1,000, 6%, 5-year, $1,000 bonds dated January 1, 2022, were...
-
As of December 31 of the current year, Petersen Corporation has prepared the following information regarding its liabilities and other obligations: Notes payable, of which $12,000 will be repaid...
-
Does log 81 (2401) = log 3 (7)? Verify the claim algebraically.
-
The irreversible gas-phase dimerization 2A A2 is carried out at 8.2 atm in a stirred contained-solids reactor to which only pure A is fed. There are 40 g of catalyst in each of the four spinning...
-
The second-order decomposition reaction A B + 2C is carried out in a tubular reactor packed with catalyst pellets 0.4 cm in diameter. The reaction is internal-diffusion-limited. Pure A enters the...
-
A first-order reaction is taking place inside a porous catalyst. Assume dilute concentrations and neglect any variations in the axial (x) direction. a. Derive an equation for both the internal and...
-
Jane Industries manufactures plastic toys. During October, Jane's Fabrication Department started work on 10,800 models. During the month, the company completed 11,800 models, and transferred them to...
-
The following balances existed in the accounting records of Jelly Candy Store Ltd. at 31 December 2019: '000 180 2,200 900 100 260 60 200 90 1,360 90 820 Development costs capitalised, 1 January 2019...
-
4 partially completed balance sheet for Blue Co. Inc. as of October 31, 2019, is presented. Where amounts are shown for various tems, the amounts are correct. Required: Jsing the following data,...

Study smarter with the SolutionInn App


