The reaction A+BC is carried out adiabatically in a constant-volume batch reactor. The rate law is -rA=k1CA1/2CB1/2-k2CC
Question:
The reaction A+B→C is carried out adiabatically in a constant-volume batch reactor. The rate law is -rA=k1CA1/2CB1/2-k2CC Plot and analyze the conversion, temperature, and concentrations of the reacting species as a function of time.
Additional information: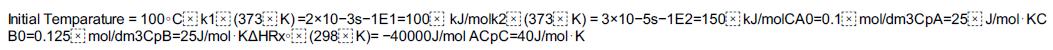
Transcribed Image Text:
Initial Temparature = 100°C k1x (373x K)=2x10-3s-1E1-100x kJ/molk2x (373x K) = 3x10-5s-1E2=150 kJ/molCA0=0.1 mol/dm3CpA=25J/mol KC B0=0.125 mol/dm3CpB=25J/mol KAHRX (298xK)= -40000J/mol ACpC=40J/mol K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Batch problem Mol balance Rate law Stoichiometry klT koexp dX Nao rav dt k2T koexp ra klCa2Cb2 k2Cc ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Use the data and reaction in Problems P11-4A and P12-7B for the following reaction: A+B C+D (a) Plot and then analyze the conversion, Q r , Q g , and temperature profiles up to a PFR reactor volume...
-
Use the data in Problem P11-4A for the following reaction. The elementary, irreversible, organic liquid-phase reaction A + B C is carried out in a flow reactor. An equal molar feed in A and B enters...
-
A reaction is to be carried out in the packed-bed reactor shown in Figure P12-19C. PFR with heat exchange. The reactants enter the annular space between an outer insulated tube and an inner tube...
-
At what points are the function. y = x tan x 2 x + 1
-
Susan Calles lived with her four daughters, Amanda, age 11,Victoria, age 5, and Jenna and Jillian, age 3. In March 1998, Calles bought an Aim N Flame utility lighter, which she stored on the top...
-
Engineering estimates show that the variable cost of manufacturing a new product will be $35 per unit. Based on market research, the selling price of the product is to be $120 per unit and variable...
-
Why is it more difficult to evaluate a universal life product than a traditional whole-life product?
-
Holly Gordon has retired and derives her income from a series of investments and a part time job at the local caf. Her income and expenses for the year include: Income $ Superannuation pension 15,000...
-
Incorrect Question 7 0/1 pts You have $600 in an account which pays 5.0% compounded annually. If you invest your money for 17 years, then how many dollars of interest will you earn by the end of the...
-
Obtain v(t) and i(t) for t > 0 in the circuit in Fig. 16.54 . 5 H ll i(t) 24 V (+ 10u(t) v(t) 200 mF
-
The elementary irreversible liquid-phase reaction A+2BC is to be carried out in a semi batch reactor in which B is fed to A. The volume of A in the reactor is 10 dm 3 , the initial concentration of A...
-
The following is an excerpt from The Morning News, Wilmington, Delaware (August 3, 1977): Investigators sift through the debris from blast in quest for the cause [that destroyed the new nitrous oxide...
-
As described in "A Word About . . . Green Chemistry: Alternative Uses for Carbohydrates" (page 484), succinic acid can be prepared enzymatically from renewable resources, such as glucose. Suggest a...
-
O The national highways agency releases information on the pro- portion of people not wearing seatbelts, aggregated by city. The data comes from random traffic stops conducted between 8am and 9am on...
-
Ivanka's Budgeted Income Statement You are the accountant for Ivanka Ltd which operates a small mixed business. The following estimates relate to the base year (Year 1): Sales of product A $100 000...
-
1. Implement the function of a XNOR gate by a 2 to 4 decoder. Use logic gates if needed at the output. 2. The following question is to design an octal to binary encoder. a) Write down the truth table...
-
# III: Worksheet 3 1. A 20 kg mass is allowed to accelerate down a frictionless 15 ramp. 20 kg 15 a. Draw a force diagram for the block. b. Determine the value of the x-component of the force of...
-
3.Baker Corporation has provided the following production and average cost data for two levels of monthly production volume. The company produces a single product Production Volume: 1,000 units:...
-
Make up a story to go with the graph at right. Be sure to interpret the x- and y-intercepts. Depth (em)
-
In July 2013, cnet.com listed the battery life (in hours) and luminous intensity (i. e., screen brightness, in cd/m2) for a sample of tablet computers. We want to know if screen brightness is...
-
Effect of pK a in the titration of weak acid with strong base. Use Equation 10-13 to compute and plot the family of curves at the left side of Figure 10-3. For a strong acid, choose a large K a ,...
-
Effect of concentration in the titration of weak acid with strong base. Use your spreadsheet from Problem 10-65 to prepare a family of titration curves for pK a = 6, with the following combinations...
-
Potassium ion in a 250.0 (0.1) mL water sample was precipitated with sodium tetraphenylborate: The precipitate was filtered, washed, dissolved in an organic solvent, and treated with excess Hg(EDTA)...
-
On December 31, 2019, Bramble Inc. borrowed $4,260,000 at 12% payable annually to finance the construction of a new building. In 2020, the company made the following expenditures related to this...
-
Shamrock Company expects to have a cash balance of $45.500 on January 1, 2022. Shamrock has budgeted the following for the first two months of the year 2022: 2. Collections from customers: January...
-
Required information Problem 1-1A (Algo) Classifying costs and computing cost per unit LO C2, P2 [The following information applies to the questions displayed below] Listed here are the costs...

Study smarter with the SolutionInn App


