Question: When oxygen is bubbled through a high temperature batch of A-containing liquid material, A oxidizes slowly to give a slowly decomposing intermediate X and final
When oxygen is bubbled through a high temperature batch of A-containing liquid material, A oxidizes slowly to give a slowly decomposing intermediate X and final product R. Here are the results of an experiment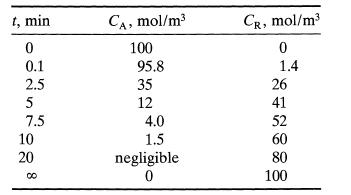
We have no way of analyzing for X; however, we are safe in assuming that at any time CA + CR + CX = CAo. What can we say about the mechanism and kinetics of this oxidation?
t, min 0 0.1 2.5 5 7.5 10 20 cc Ca, mol/m3 100 95.8 35 12 4.0 1.5 negligible 0 Cr, mol/m3 0 1.4 26 41 52 60 80 100
Step by Step Solution
3.52 Rating (159 Votes )
There are 3 Steps involved in it
To analyze the mechanism and kinetics of the oxidation reaction described we can use the principle o... View full answer

Get step-by-step solutions from verified subject matter experts


