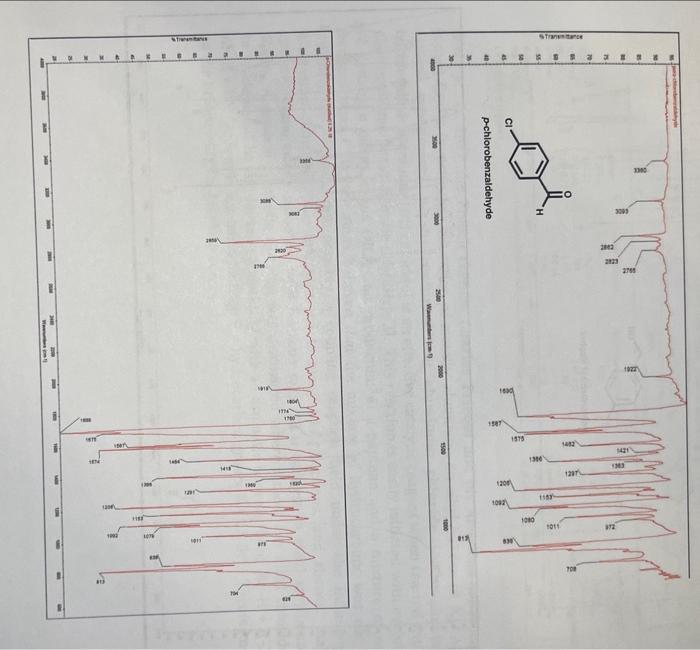
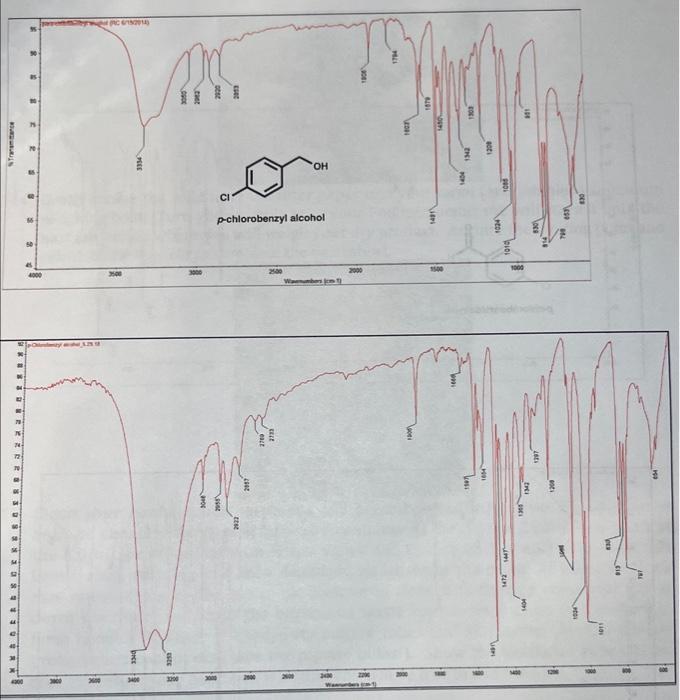
Question 5: [10 pts] In the infrared (IR) spectrum of p-chlorobenzaldehyde, a peak around 1690cm1 is lost in that of the products. This peak was representative of a (functional group). The new, large and broad peak in the product p-chlorobenzyl alcohol IR spectrum around 3300cm1 is indicative of a functional group. Use drawings of the starting material and major organic product below to circle the two functional groups. (You may also draw/highlight the peak on the IR spectrum to illustrate its appearancel) Question 6: [40 pts] Briefly describe what you did in this experiment.' Include your own observations, for example regarding the appearance of the solids (starting material and product). In CHE 232 lecture you are learning about 0xidation and Reduction reactions. The Cannizzaro Reaction is another example of a reduction reaction, in aqueous solvent. If you like, comment on the ease of this reaction to easily reduce an aldehyde. Experiment I - Crossed-Cannizzaro Reaction of p-Chlorobenzaldenyac with Formaldehyde (Synthesis of para-Chlorobenzyl alcohol via aqueous hydride [H:-] transfer) History: Cannizzaro is largely remembered for his reaction, however his greatest contribution to science was the championing of Amedeo Avogadro (1776-1856) 1811 paper that the electrolysis of water produced two volumes of hydrogen gas for each volume of oxygen gas. [Avogadro's constant NAA=6.022140857(74)1033mol1 ] Chemists finally agreed that water was actually H2O. Before this, Dalton proposed "rule of greatest simplicity" a mistaken-assertion that, since God was simple, water must be "HO7). Background: Stanislao Cannizzaro was an Italian chemist [1826-1910; b. Palermo, Italy: stud. Pisa (Piria); Univ, Genoa, Palermo, Rome] who discovered the reaction now named after him. In 1853 on his first job at the National College of Alessandria (in northern Italy), he reacted two molecules of benzaldehyde (CHsCHO) to form its corresponding carboxylic acid and alcohol. This is the original Cannizzaro reaction, where 50% of benzaldehyde is oxidized to benzoic acid and 50% reduced to benzyl alcohol; 8590% yield of benzoic acid can be isolated and benzyl alcohol in 80% yield. 2. benuldetyyal benzyl alcehol The Cannizzaro reaction of an aldehyde [with no acidic alpha () hydrogen atoms; see also Aldol reaction] is the simultaneous oxidation of one and the reduction of the other. [Oxidation = loss of electrons, or in organic chemistry: [H:]/[H]2[H][H2] : Reduction = gain of electrons.] Please review the four forms of hydrogen one may speak of: Here we may say that one molecule of benzaldehyde is oxidized to benzoic acid by the loss of a hydride and proton (equivalent to saying the loss of molecular hydrogen, or the loss of two hydrogen atoms). In the Cannizzaro Reaction mechanism (see below), an aldehyde loses a proton ( H+the nucleus of a hydrogen atom) followed by loss of a hydride ion (from its hydrate, the nucleus of a hydrogen atom and two electrons) to furnish the carboxylic acid (RCO2H). Concurrently, an aldehyde gains the hydride and then a proton to provide the 1 alcohol (RCH2OH). In the Crossed-Cannizzaro reaction, two different aldehydes are reacted [eg. pchlorobenzaldehyde (pClC6H4CHO) and formaldehyde (CH2O)]. The crossed-modification sacrifices an aldehyde (formaldehyde, in this case) to react with a more "precious" one ( p chlorobenzaldehyde) to increase the overall yield of the desired alcohol. In other words, to avoid a 50:50 mixture of desired alcohol / unwanted carboxylic acid ( 50:50 p-chlorobenzyl alcohol / p-chlorobenzoic acid). Side Note: be sure you understand the nomenclature of disubstituted benzene rings and to recognize common functional ornune. Formaldehyde (CH2O) is a toxic gas at room temperature (bp19C), so it is bubbled into water until a saturated aqueous solution is formed (37 wt.\%; 0.37mg/1mg Formalin; d25+c=1.08mg/mL). This is called "Formalin" and is more convenient form of formaldehyde (aqueous liquid rather than dealing with a noxious gas). When the gas is bubbled into water, H2O acts as a nucleophile, adds into the electrophilic carbonyl carbon of formaldehyde (Adv), and after an intermolecular proton transfer, provides the hydrate (a.ka. 1,1-diol, as it exists in solution). An acid-base reaction then ensues between formalin and potassium 2[H+]to the dianion. This dipotassium-dianion salt of formalin is then considered the active hydride donor (present in excess). (The potassium spectatorcations have been omitted in the illustration for clarity.) One of the negatively charged oxygen atoms of formalin-dianion forms a carbon-oxygen double bond (the formic acid carbonyl group) as the carbon-hydrogen bond breaks (hydrogen leaves with two electrons, "hydride"). The hydride ion is the nucleophile that adds to the electrophilic carbonyl carbon of p-chlorobenzaldehyde 1,2AdN+[H:]. This hydride transfer is the rate-determining step (RDS). monoonlon (aqueveus by byide donoen Mrecide Transtor Proton Timiter H=H (proton) (The "ipestating" potasium eations are ominad for clarify) Formalin is oxidized to formic acid (present as the potassium formate salt in alkaline solution) while p-chlorobenzaldehyde is reduced to p-chlorobenzyl alcohol (in equilibrium with its alkoxide salt and water). Once the reaction is quenched with water (or dilute acidic water), the p-chlorobenzyl alcohol (mp 6871C ) should precipitate and crystallize out of solution. This is why we use the para-chloro species, because we want to work with organic solids [benzaldehyde (bp 178C ) and benzyl alcohol (bp 205 ) are both high boiling liquids that we would not want to distii]. Qverall: Procedure: (1) Crossed-Cannizzaro Reaction: Obtain the necessary glassware and equipment. Create a hot sand bath on a stirrer-hotplate with a heat setting of 67. Then, at the analytical balances, weigh out around 155mg of solid p-chlorobenzaldehyde on tared weighing paper. Add the spin vane and white solid to the 5.0mL reaction vial. (2) Assemble your reflux condenser (if there is a long line at the balance, perform this step firstly). Be sure that the reflux tubing connected to water-faucet is fixed to the southern (bottom) port of the reflux condenser. "Water goes out the top and in the bottom." Clamp the reflux condenser to the ring stand; adjust the placement to the appropriate length/spot (you are limited with how far you can set up the experiment by how long the "exhausting" water tube is to comfortably reach the sink). You also want the reflux condenser to be centered over the stirrer-hotplate. The reflux water should be turned on for a constant drip - the water should not be a high pressure, about the same rate as a drinking bubbler ("water-fountain" for non-Massachusetts natives). Then, lightly grease the male joint of the water-jacketed reflux condenser. This is to avoid the 14/10 ground-glass joints from "freezing." The concentrated potassium hydroxide solution may dissolve glass, form potassium silicate and lock the glassware in place. Your instructor will help you with greasing the joints. The vacuum grease has been added to a syringe so you may easily apply just one little dot of grease to the joint. Wear a glove to smear it around and to avoid dirty hands. You will know if you have enough grease when you spin the reaction vial onto the reflux (3) With an automatic pipette, add the liquid components of your reaction mixture: 400 L. of methanol, 330L of formalin and 400L of freshly prepared 11M aqueous potassium hydroxide. Be sure not to cross-contaminate the automatic pipettes. (4) Join the 5.0mL reaction vial with the water-jacketed reflux condenser, tighten the screw cap to make a seal, and immediately lower the vial into the hot sand bath. Be sure to center the apparatus for ideal stir/heating. The sand bath should have a heat setting of around 67 with a similar stir rate. Dip your digital thermometer into the neck of the reflux condenser, with the probe in the neck of the reaction vial. Be sure to not seal the system! (5) In your notebook, indicate the time you begin heating and the starting temperature (on your digital thermometer). Once the reaction begins to reflux (7374C internal temperature) is when the hour countdown should begin. Record your actual temperature. (6) While heating many of the solutions will darken to a moderate brown color but some may net Record the color of your solution. Rwamnle reaction mixtures: (7) After one hour at reflux (73C) raise the apparatus out of the sand bath. Turn off your stirrer-hotplate. While the reflux water is still running, carefully remove the apparatus and use the exhaust water to cool the reaction vial over the sink. Be sure that your reaction vial is connected tightly to the reflux condenser (so that it does not accidentally fall into the sink). (8) Once the 5.0mL reaction vial is cool to the touch, remove the vlal and carefully place it on the lab bench. Turn the water off, but gently rest the reflux condenser on the tubing in the sink. You may disassemble the condenser when you have a free moment, or at the end of the experiment. (9) Remove the spin vane from the reaction vial with a pair of forceps. With a disposable graduated pipette, add 2.0mL of deionized water to the reaction vial. This will help protonate and decrease the enlubilitv of the b-chlorobenzyl alcohol product (10) Cool the reaction vial carefully in an ice-water bath (contaned in a 50mL beaker). This will help promote crystallization. With the closed-end of a melting point canillarv tube stir the contents of the reaction vial. (11) Measure the pH of the reaction muxture win pH paper. Do not dip the paper directly into the mixture. Instead, use the capillary tube (or a glass rod) to place a small amount of the solution onto the paper. (12) Allow the precipitation and crystallization to occur for 27 minutes, until it appears that the entire product has precipitated. (13) Collect your product by vacuum filtration through a Hirsch funnel. Clamp the sidearm 25mL filter flask to the ring stand, connect the tubing and then insert the Hirsch funnel (with rubber collar). Turn the vacuum on; there is no need to rinse the filter paper with water. But, be sure to use the correctly sized filter paper for your Hirsch funnel. Stir the contents of the reaction vial (solid is lazy!) and then quickly filter. Then, rinse the vial and thoroughly wash the product with 12mL of deionized water. (14) Gently scrape the solid from the filter paper into your tared (and initialed) aluminum weighing boat. Turn your sample in to your Pod instructor, she will store it until the next lab period where you will weigh your dry product. Acquire the melting point and calculate the %-yield to complete the worksheet. (15) Clean your bench space! Aqueous and organic liquid waste from this experiment must be added to the hazardous waste container in the chemical fume hoods. Clean the 5.0mL reaction vial with soapy water and agitate with a microscale test tube brush; rinse with tap and then deionized water down the drain. If water insoluble material remains on the glass, use acetone to clean it further (acetone may not go down the drain; only into the hazardous waste containers located in the chemical fume hoods.) Dispose of used/dirty culture tubes and all Pasteur pipettes into the broken glass containers (save the pipette bulbs!). Show the instructor your cleaned area and when your notebook is signed you will be free to leave the lab. Infrared Spectra: An infrared (IR) spectrum of both the starting material p-chlorobenzaldehyde and the product p-chlorobenzyl alcohol are given below. Observe the difference of stretching frequencies in the starting material compared to the product. Especially note the difference in the OH stretching region of the spectrum, and the loss of the carbonyl peak from starting material to product. Give the corresponding data to the important functional groups on your worksheet (Question 5). Use the IR Appendix in our textbook or locate a chapter on infrared spectroscopy to find typical stretching frequency ranges that correspond to organic functional groups present in a molecule. Question 5: [10 pts] In the infrared (IR) spectrum of p-chlorobenzaldehyde, a peak around 1690cm1 is lost in that of the products. This peak was representative of a (functional group). The new, large and broad peak in the product p-chlorobenzyl alcohol IR spectrum around 3300cm1 is indicative of a functional group. Use drawings of the starting material and major organic product below to circle the two functional groups. (You may also draw/highlight the peak on the IR spectrum to illustrate its appearancel) Question 6: [40 pts] Briefly describe what you did in this experiment.' Include your own observations, for example regarding the appearance of the solids (starting material and product). In CHE 232 lecture you are learning about 0xidation and Reduction reactions. The Cannizzaro Reaction is another example of a reduction reaction, in aqueous solvent. If you like, comment on the ease of this reaction to easily reduce an aldehyde. Experiment I - Crossed-Cannizzaro Reaction of p-Chlorobenzaldenyac with Formaldehyde (Synthesis of para-Chlorobenzyl alcohol via aqueous hydride [H:-] transfer) History: Cannizzaro is largely remembered for his reaction, however his greatest contribution to science was the championing of Amedeo Avogadro (1776-1856) 1811 paper that the electrolysis of water produced two volumes of hydrogen gas for each volume of oxygen gas. [Avogadro's constant NAA=6.022140857(74)1033mol1 ] Chemists finally agreed that water was actually H2O. Before this, Dalton proposed "rule of greatest simplicity" a mistaken-assertion that, since God was simple, water must be "HO7). Background: Stanislao Cannizzaro was an Italian chemist [1826-1910; b. Palermo, Italy: stud. Pisa (Piria); Univ, Genoa, Palermo, Rome] who discovered the reaction now named after him. In 1853 on his first job at the National College of Alessandria (in northern Italy), he reacted two molecules of benzaldehyde (CHsCHO) to form its corresponding carboxylic acid and alcohol. This is the original Cannizzaro reaction, where 50% of benzaldehyde is oxidized to benzoic acid and 50% reduced to benzyl alcohol; 8590% yield of benzoic acid can be isolated and benzyl alcohol in 80% yield. 2. benuldetyyal benzyl alcehol The Cannizzaro reaction of an aldehyde [with no acidic alpha () hydrogen atoms; see also Aldol reaction] is the simultaneous oxidation of one and the reduction of the other. [Oxidation = loss of electrons, or in organic chemistry: [H:]/[H]2[H][H2] : Reduction = gain of electrons.] Please review the four forms of hydrogen one may speak of: Here we may say that one molecule of benzaldehyde is oxidized to benzoic acid by the loss of a hydride and proton (equivalent to saying the loss of molecular hydrogen, or the loss of two hydrogen atoms). In the Cannizzaro Reaction mechanism (see below), an aldehyde loses a proton ( H+the nucleus of a hydrogen atom) followed by loss of a hydride ion (from its hydrate, the nucleus of a hydrogen atom and two electrons) to furnish the carboxylic acid (RCO2H). Concurrently, an aldehyde gains the hydride and then a proton to provide the 1 alcohol (RCH2OH). In the Crossed-Cannizzaro reaction, two different aldehydes are reacted [eg. pchlorobenzaldehyde (pClC6H4CHO) and formaldehyde (CH2O)]. The crossed-modification sacrifices an aldehyde (formaldehyde, in this case) to react with a more "precious" one ( p chlorobenzaldehyde) to increase the overall yield of the desired alcohol. In other words, to avoid a 50:50 mixture of desired alcohol / unwanted carboxylic acid ( 50:50 p-chlorobenzyl alcohol / p-chlorobenzoic acid). Side Note: be sure you understand the nomenclature of disubstituted benzene rings and to recognize common functional ornune. Formaldehyde (CH2O) is a toxic gas at room temperature (bp19C), so it is bubbled into water until a saturated aqueous solution is formed (37 wt.\%; 0.37mg/1mg Formalin; d25+c=1.08mg/mL). This is called "Formalin" and is more convenient form of formaldehyde (aqueous liquid rather than dealing with a noxious gas). When the gas is bubbled into water, H2O acts as a nucleophile, adds into the electrophilic carbonyl carbon of formaldehyde (Adv), and after an intermolecular proton transfer, provides the hydrate (a.ka. 1,1-diol, as it exists in solution). An acid-base reaction then ensues between formalin and potassium 2[H+]to the dianion. This dipotassium-dianion salt of formalin is then considered the active hydride donor (present in excess). (The potassium spectatorcations have been omitted in the illustration for clarity.) One of the negatively charged oxygen atoms of formalin-dianion forms a carbon-oxygen double bond (the formic acid carbonyl group) as the carbon-hydrogen bond breaks (hydrogen leaves with two electrons, "hydride"). The hydride ion is the nucleophile that adds to the electrophilic carbonyl carbon of p-chlorobenzaldehyde 1,2AdN+[H:]. This hydride transfer is the rate-determining step (RDS). monoonlon (aqueveus by byide donoen Mrecide Transtor Proton Timiter H=H (proton) (The "ipestating" potasium eations are ominad for clarify) Formalin is oxidized to formic acid (present as the potassium formate salt in alkaline solution) while p-chlorobenzaldehyde is reduced to p-chlorobenzyl alcohol (in equilibrium with its alkoxide salt and water). Once the reaction is quenched with water (or dilute acidic water), the p-chlorobenzyl alcohol (mp 6871C ) should precipitate and crystallize out of solution. This is why we use the para-chloro species, because we want to work with organic solids [benzaldehyde (bp 178C ) and benzyl alcohol (bp 205 ) are both high boiling liquids that we would not want to distii]. Qverall: Procedure: (1) Crossed-Cannizzaro Reaction: Obtain the necessary glassware and equipment. Create a hot sand bath on a stirrer-hotplate with a heat setting of 67. Then, at the analytical balances, weigh out around 155mg of solid p-chlorobenzaldehyde on tared weighing paper. Add the spin vane and white solid to the 5.0mL reaction vial. (2) Assemble your reflux condenser (if there is a long line at the balance, perform this step firstly). Be sure that the reflux tubing connected to water-faucet is fixed to the southern (bottom) port of the reflux condenser. "Water goes out the top and in the bottom." Clamp the reflux condenser to the ring stand; adjust the placement to the appropriate length/spot (you are limited with how far you can set up the experiment by how long the "exhausting" water tube is to comfortably reach the sink). You also want the reflux condenser to be centered over the stirrer-hotplate. The reflux water should be turned on for a constant drip - the water should not be a high pressure, about the same rate as a drinking bubbler ("water-fountain" for non-Massachusetts natives). Then, lightly grease the male joint of the water-jacketed reflux condenser. This is to avoid the 14/10 ground-glass joints from "freezing." The concentrated potassium hydroxide solution may dissolve glass, form potassium silicate and lock the glassware in place. Your instructor will help you with greasing the joints. The vacuum grease has been added to a syringe so you may easily apply just one little dot of grease to the joint. Wear a glove to smear it around and to avoid dirty hands. You will know if you have enough grease when you spin the reaction vial onto the reflux (3) With an automatic pipette, add the liquid components of your reaction mixture: 400 L. of methanol, 330L of formalin and 400L of freshly prepared 11M aqueous potassium hydroxide. Be sure not to cross-contaminate the automatic pipettes. (4) Join the 5.0mL reaction vial with the water-jacketed reflux condenser, tighten the screw cap to make a seal, and immediately lower the vial into the hot sand bath. Be sure to center the apparatus for ideal stir/heating. The sand bath should have a heat setting of around 67 with a similar stir rate. Dip your digital thermometer into the neck of the reflux condenser, with the probe in the neck of the reaction vial. Be sure to not seal the system! (5) In your notebook, indicate the time you begin heating and the starting temperature (on your digital thermometer). Once the reaction begins to reflux (7374C internal temperature) is when the hour countdown should begin. Record your actual temperature. (6) While heating many of the solutions will darken to a moderate brown color but some may net Record the color of your solution. Rwamnle reaction mixtures: (7) After one hour at reflux (73C) raise the apparatus out of the sand bath. Turn off your stirrer-hotplate. While the reflux water is still running, carefully remove the apparatus and use the exhaust water to cool the reaction vial over the sink. Be sure that your reaction vial is connected tightly to the reflux condenser (so that it does not accidentally fall into the sink). (8) Once the 5.0mL reaction vial is cool to the touch, remove the vlal and carefully place it on the lab bench. Turn the water off, but gently rest the reflux condenser on the tubing in the sink. You may disassemble the condenser when you have a free moment, or at the end of the experiment. (9) Remove the spin vane from the reaction vial with a pair of forceps. With a disposable graduated pipette, add 2.0mL of deionized water to the reaction vial. This will help protonate and decrease the enlubilitv of the b-chlorobenzyl alcohol product (10) Cool the reaction vial carefully in an ice-water bath (contaned in a 50mL beaker). This will help promote crystallization. With the closed-end of a melting point canillarv tube stir the contents of the reaction vial. (11) Measure the pH of the reaction muxture win pH paper. Do not dip the paper directly into the mixture. Instead, use the capillary tube (or a glass rod) to place a small amount of the solution onto the paper. (12) Allow the precipitation and crystallization to occur for 27 minutes, until it appears that the entire product has precipitated. (13) Collect your product by vacuum filtration through a Hirsch funnel. Clamp the sidearm 25mL filter flask to the ring stand, connect the tubing and then insert the Hirsch funnel (with rubber collar). Turn the vacuum on; there is no need to rinse the filter paper with water. But, be sure to use the correctly sized filter paper for your Hirsch funnel. Stir the contents of the reaction vial (solid is lazy!) and then quickly filter. Then, rinse the vial and thoroughly wash the product with 12mL of deionized water. (14) Gently scrape the solid from the filter paper into your tared (and initialed) aluminum weighing boat. Turn your sample in to your Pod instructor, she will store it until the next lab period where you will weigh your dry product. Acquire the melting point and calculate the %-yield to complete the worksheet. (15) Clean your bench space! Aqueous and organic liquid waste from this experiment must be added to the hazardous waste container in the chemical fume hoods. Clean the 5.0mL reaction vial with soapy water and agitate with a microscale test tube brush; rinse with tap and then deionized water down the drain. If water insoluble material remains on the glass, use acetone to clean it further (acetone may not go down the drain; only into the hazardous waste containers located in the chemical fume hoods.) Dispose of used/dirty culture tubes and all Pasteur pipettes into the broken glass containers (save the pipette bulbs!). Show the instructor your cleaned area and when your notebook is signed you will be free to leave the lab. Infrared Spectra: An infrared (IR) spectrum of both the starting material p-chlorobenzaldehyde and the product p-chlorobenzyl alcohol are given below. Observe the difference of stretching frequencies in the starting material compared to the product. Especially note the difference in the OH stretching region of the spectrum, and the loss of the carbonyl peak from starting material to product. Give the corresponding data to the important functional groups on your worksheet (Question 5). Use the IR Appendix in our textbook or locate a chapter on infrared spectroscopy to find typical stretching frequency ranges that correspond to organic functional groups present in a molecule