A hydrochloric acid solution will neutralize a sodium hydroxide solution. Look at the molecular views showing one
Question:
A hydrochloric acid solution will neutralize a sodium hydroxide solution. Look at the molecular views showing one beaker of HCl and four beakers of NaOH. Which NaOH beaker will just neutralize the HCl beaker? Begin by writing a balanced chemical equation for the neutralization reaction.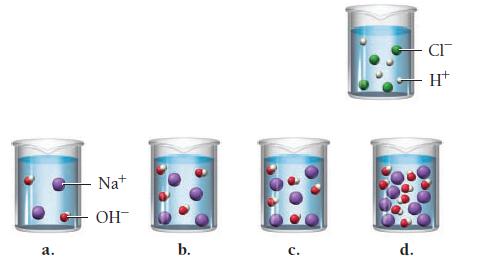
Transcribed Image Text:
a. Na+ OH™ b. C. d. CI H+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
A 0.20 M solution of hydrochloric acid (HCl) is mixed with a 0.10 M solution of sodium hydroxide (NaOH) in a 1.0 L container. a) Write the balanced chemical equation for the reaction that occurs when...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
1.) Find the equation of a polynomial in standard form of lowest degree possible that crosses the x-axis at x = 1, bounces off of the x-axis at x = -1, has a y-intercept of 4, goes to co as x goes to...
-
What is a steering committee? Discuss its role in a systems study performed by a consulting firm.
-
Which is not an example of a batch total? a. Record count b. Financial total c. Hash total d. Exception total
-
Do you think Allisha should support Michelles recommendation that Lani be suspended? Why or why not?
-
Construction Forms Corporation buys securities to be available for sale when circumstances warrant, not to profit from short-term differences in price and not necessarily to hold debt securities to...
-
Carla Vista Company has gathered the following information. Units in beginning work in process 0 Units started into production 3 4 , 5 6 0 Units in ending work in process 5 , 7 6 0 Percent complete...
-
Consider the following pseudo-WEP protocol. The key is 4 bits and the IV is 2 bits . The IV is appended to the end of the key when generating the keystream. Suppose that the shared secret key is 10...
-
Review the solubility rules. Without referring back to the rules, have each group member list two ionic compounds that are expected to be soluble and two that are expected to be insoluble. Include at...
-
Consider the generic ionic compounds with the formulas A 2 X and BY 2 and the following solubility rules: A 2 X soluble; BY 2 soluble; AY insoluble; BX soluble. Assume A+ ions are circles, B 2 + ions...
-
Jackson Corporation purchased shares of Phillips Corporation in the following sequence: The book value of Phillips's net assets at January 1, 20X6, was \(\$ 200,000\). Each year since Jackson first...
-
At the Business Level there are a couple main strategies that companies use- Cost Leadership and Differentiation. What is the difference between them? Share some examples of companies or specific...
-
https://youtu.be/c_Eutci7ack After watching the video, what are your thoughts on Power? Would you want to have this Power ? Why would you not want this Power? If you are a manager or want to be a...
-
Find anti derivative of ( 2 t - 4 + 3 ^ ( 1 / 2 ) ) / t ^ ( 1 / 2 )
-
How Adidas is using creative narratives to build brand equity Adidas' outdoor division is drawing on the expertise of its wider athletic business while at the same time flexing its creative muscle to...
-
May I have a word" Alysha Stark popped her head in at the corner office of the Managing Director Mike O' Connor. It's early on a Monday morning. When Alysha, his star Director, starts something this...
-
In Figure 9.38 is shown the pressure-temperature phase diagram for H2O. Apply the Gibbs phase rule at points A, B, and C; that is, specify the number of degrees of freedom at each of the points-that...
-
The Taylor's series expansion for cosx about x = 0 is given by: where x is in radians. Write a user-defined function that determines cosx using Taylor's series expansion. For function name and...
-
Using the results of Problems 1.631.65, generate a statement about the general design approach to achieving a very stiff system.
-
Calculate the weight of 1 m 3 of kerosene if it has a mass of 825 kg.
-
Assume that a man weighs 160 lb (force). a. Compute his mass in slugs. b. Compute his weight in N. c. Compute his mass in kg.
-
If the month-end bank statement shows a balance of $75,000, outstanding checks are $54,000, a deposit of $15,000 was in transit at month end, and a check for $4,000 was erroneously charged by the...
-
SECTION A [100 MARKS] Answer ALL questions in this section. QUESTION 1 Explain the difference between financial and management accounting.
-
If Donald obtained a business loan of $270,000.00 at 5.34% compounded semi- annually, how much should he pay at the end of every 6 months to clear the loan in 25 years? Round to the nearest cent

Study smarter with the SolutionInn App


