A meteor contains 0.556 g of Pb-206 to every 1.00 g of U-238. Assuming that the meteor
Question:
A meteor contains 0.556 g of Pb-206 to every 1.00 g of U-238. Assuming that the meteor did not contain any Pb-206 at the time of its formation, determine the age of the meteor. Uranium-238 decays to lead-206 with a half-life of 4.5 billion years.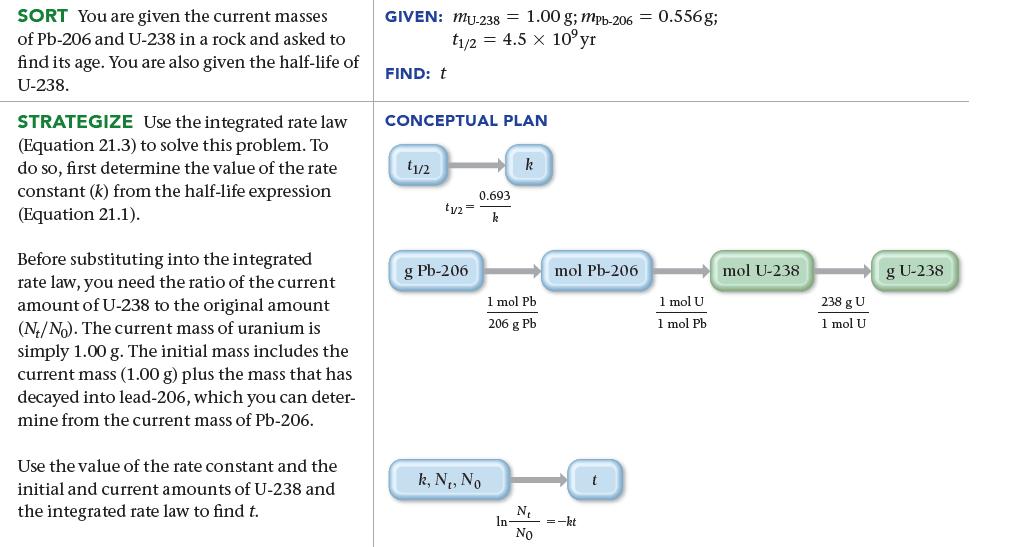
Transcribed Image Text:
SORT You are given the current masses of Pb-206 and U-238 in a rock and asked to find its age. You are also given the half-life of U-238. STRATEGIZE Use the integrated rate law (Equation 21.3) to solve this problem. To do so, first determine the value of the rate constant (k) from the half-life expression (Equation 21.1). Before substituting into the integrated rate law, you need the ratio of the current amount of U-238 to the original amount (N/No). The current mass of uranium is simply 1.00 g. The initial mass includes the current mass (1.00 g) plus the mass that has decayed into lead-206, which you can deter- mine from the current mass of Pb-206. Use the value of the rate constant and the initial and current amounts of U-238 and the integrated rate law to find t. GIVEN: MU-238 = 1.00 g; mpb-206 = 0.556g; t1/2 = 4.5 x 10°yr FIND: t CONCEPTUAL PLAN 11/2 tu= g Pb-206 0.693 k k, N₁, No k 1 mol Pb 206 g Pb In- N₁ No mol Pb-206 =-kt t 1 mol U 1 mol Pb. mol U-238 238 g U 1 mol U g U-238
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
t12 0693 k 0693 t12 154 x 100yr k 5 Nt In No ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The contents of the rock have a 206Pb to 238U mass ratio of 0.175:1.00. Assuming that the rock did not contain any 206Pb at the time of its formation, determine the age of the rock. Uranium-238...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Sketch the following regions and write an iterated integral of a continuous function f over the region. Use the order dy dx. R = {(x, y): 0 x 4, x y 8x}
-
Web Design Services billed its customers a total of $490,200 for the month of August, including 9 percent federal excise tax and 5 percent sales tax. 1. Determine the proper amount of service revenue...
-
Based on the case information provided, describe specifically how WorldCom violated the matching principle. In your description, please identify a journal entry that may have been used by WorldCom to...
-
Indicate the four types of price competitive levels.
-
Queen Savings is attempting to determine its liquidity requirements today (the last day in August) for the month of September. September is usually a month of heavy loan demand due to the beginning...
-
During the month of March, Sandhill Co's employees earned wages of $68,000. Withholdings related to these wages were $5,202 for FICA $8,160 for federal income tax, $3,400 for state income tax, and...
-
Consider this graph representing the decay of a radioactive nuclide. What is the half-life of the nuclide? (a) 625 years (b) 1250 years (c) 2500 years (d) 3125 years
-
The chart shows the mass of a decaying nuclide versus time. What is the half-life of the decay? a) 15 min b) 25 min c) 35 min d) 70 min Mass Nuclide (g) 30- 25 DO 20- 15 10 5 0 0 20 40 60 80 Time...
-
Fill in the blank with an appropriate word, phrase, or symbol(s). The art and science of gathering, analyzing, and making inferences (predictions) from numerical information obtained in an experiment...
-
Use the Comparison Theorem to determine whether the integral is convergent or divergent. L da
-
Problem 3 (2 scenarios) Scenario 1: Rocky Inc hired a new intern from CSU to help with year-end inventory. The intern computed the inventory counts at the end of 2020 and 2021. However, the intern's...
-
A CM reactor receives influent containing 10.0 mg/L of tracer for 2 h. Then tracer addition is terminated but the flow remains steady. The volume of the reactor is 10 L and the flow rate is 2 L / h....
-
Solve the given system of equations graphically by using a graphing calculator. y=5x x+y2=81 Find the solution with the smaller x-value. x= y= (Type an integer or a decimal rounded to one decimal...
-
I-The market for Sony's Playstation5 game console has changed from 2021 to 2023. With restrictions from the Covid-19 pandemic ending people are finding other entertainment options available such as...
-
During the most recent year, Ogden Co. bought 2,800 shares of Dublin common stock at $35, 590 shares of Chile stock at $45.50, and 1,000 shares of Russian stock at $70 - all as available-for-sale...
-
Clark, PA, has been engaged to perform the audit of Kent Ltd.s financial statements for the current year. Clark is about to commence auditing Kents employee pension expense. Her preliminary enquiries...
-
A thin plastic rod 20 cm long carries 3.2 nC distributed uniformly over its length. (a) If the rod is bent into a ring, find the potential at its center. (b) If its bent into a semicircle, find the...
-
A thin ring of radius R carries charge 3Q distributed uniformly over three-fourths of its circumference, and -Q over the rest. Find the potential at the rings center.
-
The potential at the center of a uniformly charged ring is 45 kV, and 15 cm along the ring axis the potential is 33 kV. Find the rings radius and total charge.
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?

Study smarter with the SolutionInn App


