A weak unknown monoprotic acid is titrated with a strong base. The titration curve is shown. Find
Question:
A weak unknown monoprotic acid is titrated with a strong base.
The titration curve is shown. Find Ka for the unknown acid.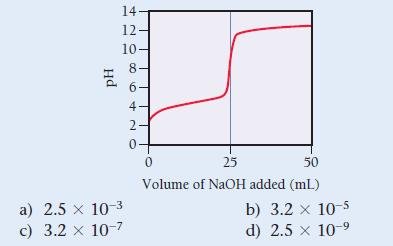
Transcribed Image Text:
14. 12. 10 Hd ∞0 60+ NO 8 a) 2.5 x 10-3 c) 3.2 x 10-7 6- 4- 2- 0+ 0 25 50 Volume of NaOH added (ml) b) 3.2 x 10-5 d) 2.5 x 10-9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
b ...View the full answer

Answered By

Madhvendra Pandey
Hi! I am Madhvendra, and I am your new friend ready to help you in the field of business, accounting, and finance. I am a College graduate in B.Com, and currently pursuing a Chartered Accountancy course (i.e equivalent to CPA in the USA). I have around 3 years of experience in the field of Financial Accounts, finance and, business studies, thereby looking forward to sharing those experiences in such a way that finds suitable solutions to your query.
Thus, please feel free to contact me regarding the same.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The figure compares the titration of a monoprotic weak acid with a monoprotic weak base and the titration of a diprotic acid with strong base. (a) Write the reaction between the weak acid and the...
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
A weak monoprotic acid is titrated with 0.100 M NaOH. It requires 50.0 mL of the NaOH solution to reach the equivalence point. After 25.0 mL of base is added, the pH of the solution is 3.62. Estimate...
-
How are writable CDs implemented?
-
Judy Jean, a recent graduate of Rollings accounting program, evaluated the operating performance of Artie Companys six divisions. Judy made the following presentation to Arties board of directors and...
-
A ballistic pendulum is a device for measuring bullet speeds. One of the simplest versions consists of a block of wood hanging from two long cords. (Two cords are used so that the bottom face of the...
-
What a job description and job specifications are and how they are used in human resource practices
-
The following is the alphabetical adjusted trial balance of the Meadows Company on December 31, 2007: Required Prepare the December 31, 2007 balance sheet of the Meadows Company. Compute the...
-
Which of the following strategies would reduce the number of units required to be sold to break-even? Group of answer choices: Take advantage of bulk discounts from suppliers. All of these strategies...
-
A solution containing lead(II) nitrate is mixed with one containing sodium bromide to form a solution that is 0.0150 M in Pb(NO 3 ) 2 and 0.00350 M in NaBr. Does a precipitate form in the newly mixed...
-
Determine whether each compound is more soluble in an acidic solution than it is in a neutral solution. (a) BaF 2 (b) AgI (c) Ca(OH) 2
-
Visit the FBIs web site and review a posting entitled 2009 Financial Crimes Report. Read the Forensic Accountant Program section and prepare a memo to your instructor summarizing your findings.
-
Suppose that Angelina and Brad own the only two professional photography stores in town. Each must choose between a low price and a high price for senior photo packages. The annual economic profit...
-
based on the article How Chili's Is Prepping for Tough Times, Starting With the Fries by Heather Haddon. What is corporate social responsibility, and what is one way that Chili's can better pursue...
-
QUESTION 2 (20 marks) CLO 5 a. Explain what the following ratios indicate to a firm: (i) Acid Test Ratio (ii) Return on Capital Employed (ROCE) (iii) Debtors Collection Period (iv) Working Capital (2...
-
Lazlo s estimates uncollectible accounts to be 0 . 9 % of sales. Its year - end unadjusted trial balance shows Accounts Receivable of $ 1 1 2 , 5 0 0 and sales of $ 9 6 5 , 0 0 0 . If Lazlo s uses...
-
Identify one or two of the best and one or two of the worst work teams on which you served as a member. 1. Identify the top three to five factors that made the team the best or the worst in terms of...
-
Three loads are connected in parallel to a 1200 V rms source. Load 1 absorbs 60 kVAR at pf = 0.85 lagging, load 2 absorbs 90 kW and 50 kVAR leading, and load 3 absorbs 100 kW at pf = 1. (a) Find the...
-
Evaluate the integral, if it exists. Jo y(y + 1) dy
-
The magnitude plot in Fig. 14.76 represents the transfer function of a preamplifier. Find H(s). H (dB) 20 20 dB/decade 2,122 20 dB/decade 50 500
-
The Bode magnitude plot of H(Ï) is shown in Fig. 14.75 . Find H(Ï). H (dB) A 20 0.1 @ (rad/s) 10 +20 dB/decade -40 dB/decade
-
Find the transfer function H(Ï) with the Bode magnitude plot shown in Fig. 14.74 . H (dB) -20 dB/decade 20 -20 @ (rad/s) 2 20 100
-
A company has EBIT of $250,000 in perpetuity. Its cost of debt and equity at different levels of debt is given below. The tax rate is 35%. Calculate the values of A, B, C, and D. Debt Kd Ke...
-
solve full question please EX-9B On February 1, 2011. Giant Corp. issued an 5800,000, 5%, two-year bond. Interest is payable quarterly each May 1, August 1, November 1, and February 1. Required Part...
-
In which transaction cycle would information for retiring long-term debt be most likely to pass between internal and external accounting information systems. Select one: A. the financing cycle B. the...

Study smarter with the SolutionInn App


