An aluminum sphere contains 8.55 * 10 22 aluminum atoms. What is the spheres radius in centimeters?
Question:
An aluminum sphere contains 8.55 * 1022 aluminum atoms. What is the sphere’s radius in centimeters?
The density of aluminum is 2.70 g/cm3.

Transcribed Image Text:
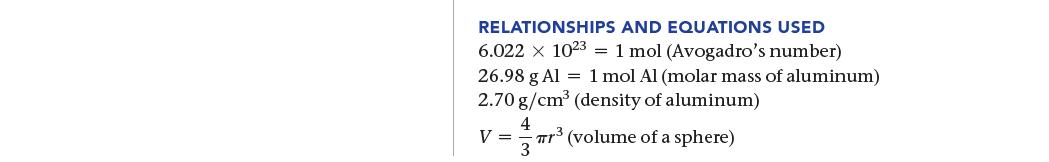
SORT You are given the number of aluminum atoms in a sphere and the density of aluminum. You are asked to find the radius of the sphere. STRATEGIZE The heart of this problem is density, which relates mass to volume; though you aren't given the mass directly, you are given the number of atoms, which you can use to find mass. 1. Convert from number of atoms to number of moles using Avogadro's number as a conver- sion factor. 2. Convert from number of moles to mass using molar mass as a conversion factor. 3. Convert from mass to volume (in cm³) using density as a conversion factor. 4. Once you calculate the volume, find the radius from the volume using the formula for the volume of a sphere. GIVEN: 8.55 x 10²2 Al atoms d = 2.70 g/cm³ FIND: radius (r) of sphere CONCEPTUAL PLAN Number of Al atoms 1 mol Al 6.022 x 1023 Al atoms V (in cm³) V = mol Al πTr³ 26.98 g Al 1 mol Al g Al 1 cm³ 2.70 g Al V (in cm³)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
855 x 1022 Al atoms X 4 3 V ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
What is the company' s sustainable growth rate? Suppose the firm targets a 30% sales growth rate next year, and the firm doesn't want to issue new equity, what will be the the firm's new D/A ratio?...
-
The Ebitts Field Corp. manufactures baseball gloves. Charlie Botz, the companys top salesman, has recommended expanding into the baseball bat business. He has put together a project proposal...
-
On July 1,the first day of their fiscal year, the City of Denver sold bonds with a face value of $10,000,000 at 102 percent par. The bonds bear annual interest at 6 percent; interest is payable...
-
Cede & Co. can borrow at 9 percent. Cede currently has no debt, and the cost of equity is 16 percent. The current value of the firm is $540,000. What will the value be if Cede borrows $110,000 and...
-
A mechanical system is shown in Figure P2.17, which is subjected to a known displacement x3(t) with respect to the reference, (a) Determine the two independent equations of motion. (b) Obtain the...
-
Explain what it means for a forward currency to sell at a discount and at a premium. AppendixLO1
-
Information on Hanleys direct labor costs for January 2010 is as follows: Actual direct labor rate ........... $7.50 Standard direct labor hours allowed ....... 11,000 Actual direct labor hours...
-
Part 2: Adjusting Entries Advance Corporation has the following transactions that require adjustments for March, 2017 Data for adjusting entries 1- March 1 Received $2,400 in advance for a work to be...
-
Use the data in COUNTYMURDERS to answer this question. Use only the year 1996. The variable murders is the number of murders reported in the county. The variable execs is the number of executions...
-
Write the symbol for each element and classify it as a metal, nonmetal, or metalloid. a. Gold b. Fluorine c. Sodium d. Tin e. Argon
-
A mixture of CaCO 3 and (NH 4 ) 2 CO 3 is 61.9% CO 3 by mass. Find the mass percent of CaCO 3 in the mixture.
-
Give the ground-state electron configuration and number of unpaired electrons expected for each of the following ions: (a) Fe 3+ ; (b) Bi 3+ ; (c) Si 4+ ; (d) I 2 .
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Solve the following linear system by Gaussian elimination with back-substitution without introducing fractions in your row-reduction. If there is no solution, explain why. -3x+8y + 82 = -8 -2x+ y -...
-
Introduction Some predictions are a slam dunk. Retail will continue to be driven by technology. Science fiction is coming to life in the form of robotics and virtual reality. And the Internet will...
-
Oswego Clay Pipe Company provides services of $ 5 0 , 0 0 0 to Southeast Water District # 4 5 on April 1 2 of the current year with terms 1 / 1 5 , n / 6 0 . What would Oswego record on April 1 2 ?...
-
Assume the following excerpts from a company's balance sheet: Property, plant, and equipment Beginning Balance $3,500,000 Ending Balance $3,700,000 $1,100,000 $800,000 Long-term investments During...
-
What is the function of a centromere? At what stage of the cell cycle would you expect the centromere to be the most important?
-
For the given transfer function: Vo(s) / Vi(s) = (s^2C^2R^2 + 1) / (s^2C^2R^2 + 4sCR + 1) Assumiing that 1/(CR) = 120 PI so write the matlab code to find the magnitude plot
-
Acid-catalyzed hydration of 1-methylcyclohexene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explane.
-
Calculate S surroundings and S total for part (c) of Problem P5.6. Is the process spontaneous? The state of the surroundings is T = 310.K, P = 0.333 bar.
-
Acid-catalyzed hydration of 1-methylcyclohexene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explane.
-
On January 1, 20X1, Toy inc. Issued $500,000 of convertible bonds. The bonds mature on December 31, 20X5, Interest is payable annually at 6.0% on December 31. The bonds are convertible at the...
-
Exercise 18-11 (Algo) Balance sheet identification and preparation LO P1 End-of-year current assets for two different companies follow. One is a manufacturer, Rayzer Skis Manufacturing, and the...
-
The duties or restrictions stated in Circular 230, Subpart B, do NOT include __________. Advertising that taxpayers may use pay stubs in lieu of Form W-2. Charging exorbitant fees. Knowingly...

Study smarter with the SolutionInn App


