An electrochemical cell is based on these two half-reactions: Calculate the cell potential at 25 C. Ox:
Question:
An electrochemical cell is based on these two half-reactions: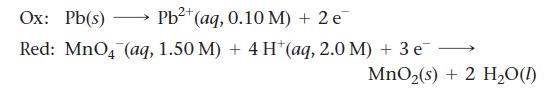
Calculate the cell potential at 25 °C.
Transcribed Image Text:
Ox: Pb(s) Pb²+ (aq, 0.10 M) + 2 e Red: MnO4 (aq, 1.50 M) + 4H* (aq, 2.0 M) + 3 e MnO₂ (s) + 2 H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

BRIAN MUSINGA
I possess a Bachelors of Commerce degree(Marketing option) and am currently undertaking an MBA in marketing. I believe that I possess the required knowledge and skills to tutor in the subject named. I have also written numerous research academic papers much to the satisfaction of clients and my professors.
5.00+
2+ Reviews
17+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An electrochemical cell is constructed such that on one side a pure nickel electrode is in contact with a solution containing Ni2+ ions at a concentration of 3 10-3 M. The other cell half consists...
-
The reaction taking place in an electrochemical cell under standard conditions is Fe 2+ (aq) + Ag + (aq) Fe 3+ (aq) + Ag(s) a. Write two half-equations for this reaction. For each, state whether...
-
An electrochemical cell is based on the following two half-reactions: Ox: Pb(s)Pb2+(aq,Pb(s)Pb2+(aq, 0.15 MM )+2e)+2e Red: MnO4(aq,MnO4(aq, 1.80 MM )+4H+(aq,)+4H+(aq, 1.9 MM )+3e)+3e MnO2(s)+2H2O(l)...
-
Stock W, X and Y have expected returns of 9.0%, 16.1% and 11.3% respectively. Based on this and the attached information, what is the expected return of your portfolio? Stock W X Y Number of Shares...
-
Last year, Biomed Laboratories, inc., researched and perfected a cure for the common cold. Called Cold-Gone, the product sells for $28.00 per package, each of which contains five tablets. Standard...
-
To help you decide which of your two current suppliers deserves the larger contract next year, you have rated a random sample of plastic cases from each one. The data are a composite of several...
-
5. Assume that the volatility of the S&P index is 30%. a. What is the price of a bond that after 2 years pays S0 + max(0, S2 S0)? b. Suppose the bond pays S0 + [ max(0, S2 S0)] in year 2. For what...
-
For the past several years, Samantha Hiogan has operated a part-time consulting business from her home. As of July 1, 20Y9, Samantha decided to move to rented quarters and to operate the business,...
-
variance analysis
-
Go to the following World Bank webpage: http://www.app.collinsindicate.com/worldbankatlas-global/en-us In the search box in the upper right corner of the page, enter the following: GDP per capita,...
-
A voltaic cell employs the following redox reaction: Calculate the cell potential at 25 C under each set of conditions. Sn+ (aq) + Mn(s) 2+ Sn(s) + Mn+ (aq)
-
Refer to the pizza store location x2 data on pages 522524. What statistical decisions could be made if the 0.01 significance level were selected rather than the 0.05 level?
-
1. create a concept map for 0D, 1D, 2D and 3D crystals 2. write down the formulas for quantifying numbers of defects
-
\fNOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF AMERICAN AIRLINES GROUP INC . Commitments , Contingencies and Guarantees ( 2 ) Aircraft and Engine Purchase Commitment Under all of our aircraft and...
-
Critical Values. In Exercises 41-44, find the indicated critical value. Round results to two decimal places. 41. Z0.25 42. Z0.90 43. Z0.02 44. 20.05
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
In todays social and business environments, some organizations only talk the talk regarding ethics and ethical conduct rather than walk the ethical organizational path. In what ways can ethical and...
-
Presented here are summarized data from the balance sheets and income statements of Wiper. Inc.: Required: a. Calculate return on investment, based on net income and average total assets, for 2017...
-
Write the general quadratic equation y2 - 8y - 4x + 28 = 0 in standard form. Determine the vertex, focus, and directrix of the parabola defined by this equation. Sketch a graph.
-
What would the pattern look like for a laserbeam diffracted by the three crossed gratings of Fig. P.13.39? Fig. P.13.39?
-
Make a rough sketch of the Fraunhofer diffraction pattern that would arise if a transparency of Fig. P.13.40a served as the object. How would you filter it to get Fig. P.13.40b? Fig. P.13.40a and...
-
Repeat the previous problem using Fig. P.13.41 instead. previous problem Make a rough sketch of the Fraunhofer diffraction pattern that would arise if a transparency of Fig. P.13.40a served as the...
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


